1295
Age Dependent Changes of Water Exchange Rate in Blood Brain Barrier (BBB) Assessed by Diffusion-Prepared Arterial Spin Labeling1Laboratory of Functional MRI Technology (LOFT), Stevens Neuroimaging and Informatics Institute, University of Southern California, Los Angeles, CA, United States, 2Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA, United States, 3Department of Ophthalmology, Keck School of Medicine, University of Southern California, Los Angeles, CA, United States
Synopsis
The purpose of this study was to investigate the relationship between water exchange rate (kw) of blood brain barrier and age. Diffusion Prepared pCASL technique was used to measure kw in four datasets with a wide range of age (8 to 81 years). Global and regional kw values as well as cerebral blood flow and arterial transit time were analyzed. The results show an overall trend of decreasing kw with age, in parietal and parahippocampal regions there are U-shaped and inverted U-shaped changes of kw with age.
Introduction
Blood-brain-barrier (BBB) plays a critical role in the delivery of nutrients and oxygen in the brain. Previous studies have reported that BBB permeability increases with age and may be related to microvascular diseases and dementia1. BBB permeability is usually measured by dynamic contrast enhanced (DCE) MRI with the injection of Gd based contrast agents. Recently, diffusion prepared pseudo-continuous arterial spin labeling (DP-pCASL) has been developed to assess the water exchange rate (kw) across BBB without contrast2. In this study, we analyzed DP-pCASL data collected on a wide range of age groups (8 to 81 years) to investigate age dependent changes of BBB water exchange rate.Methods
1. MRI imaging protocolThe DP-pCASL sequence described in2 was applied with imaging parameters: resolution = 3.5×3.5×8 mm3, TR = 4200ms, TE = 36.22ms, field-of-view (FOV) of 224 mm, slice number = 12. Labeling duration was 1500 ms and post labeling delays (PLDs) were 900ms and 1800ms. The first PLD was used to estimate arterial transit time (ATT). The second PLD was implemented with 2 diffusion encoding b values: 0 and 50 s/mm2 to estimate the kw. Cerebral blood flow (CBF) was quantified from the perfusion signals acquired at 1800ms PLD without diffusion preparation. The total scan time was about 10 minutes.
2. Dataset collection
We included four datasets in different age groups (total N=61, 36 males): 1) Pediatric subjects: N=9, age range=8 to 17, mean=13±2.9 years, 6 males; 2) Young adults: N=5, age range=23 to 29, mean=25.2 ± 2.5 years, 4 males; 3) Elderly Latinx subjects: N=8, age range=64 to 70, mean=67.6±3.6 years, 2 males; 4) Elderly African American subjects: N=39, age range=40 to 81, mean=63.9±10.2 years, 24 males. All subjects were scanned on the same Siemens 3T Prisma scanner.
3. Postprocessing and analysis
The custom Matlab toolbox with total-generalized-variation (TGV) regularized single-pass-approximation (SPA) model was used to calculate CBF, ATT and kw maps in each subject. The M0 image and parametric maps were then normalized to the MNI template using SPM12. Regional analysis was performed using the AAL template including large cortical ROIs including frontal cortex, parietal cortex, temporal cortex, anterior and posterior cingulate cortex and precuneus, and subcortical structures including amygdala, caudate and putamen, hippocampus and parahippocampal regions. The mean values of CBF, ATT and kw in each ROI and whole brain were extracted and regressed with age and gender. Nonlinear relationship of CBF, ATT and kw with age was explored by including age square in regression model.
Results
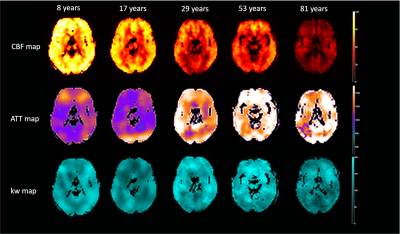
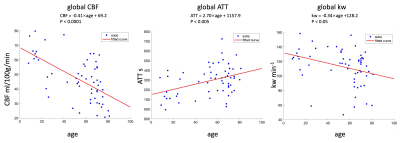
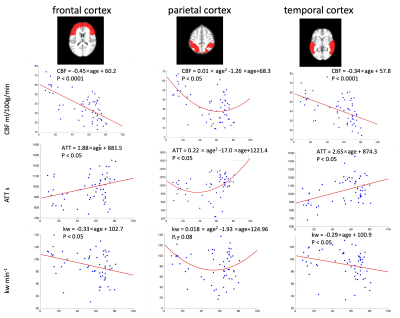
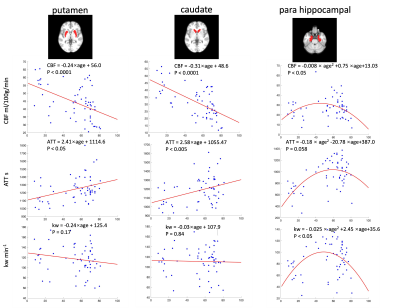
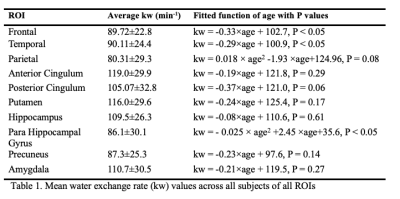
Figure 1 shows the CBF, ATT and kw maps of five representative subjects across the whole age range. Figure 2 shows the scatterplots of global mean CBF, ATT and kw values versus age. Global mean CBF decreases, ATT increases, and kw decreases with age. Gender was not significant in all our analyses, although CBF was higher, kw was lower in females than males. Figure 3 and 4 show regional analyses of cortical and subcortical structures. Frontal and temporal cortices follow the linear trends of global values. However, a U-shaped relationship with age was observed in the parietal cortex, while an inverted U-shaped relationship with age was observed in the parahippocampal regions (Table 1).Discussion
In this study, we studied age-related effects of CBF, ATT and kw in a wide range of age groups using DP-pCASL. A significant trend of decreasing CBF and increasing ATT with age was found, which is consistent with previous studies3. We also found a decreasing trend of BBB water exchange rate with age. Water exchange across the BBB is maintained at a high rate in a healthy brain, which has been implicated in the glymphatic system and clearance of brain waste. With aging, this function deteriorates, leading to decreasing kw with age. Our finding is consistent with a recent study that reported positive associations of kw with CSF amyloid-beta42 and episodic memory score in a cohort of aged subjects with normal cognition4. Previous studies reported complex age and gender dependent changes of CBF across brain regions5, which may be related to the U-shaped and inverted U-shaped curves of kw vs. age observed in parietal and parahippocampal regions in this study.There are a few limitations of our study: 1) The two elderly cohorts consist of minority groups while the pediatric and young adult groups are mixed; 2) There were few mid-aged subjects; 3) Partial volume correction was not performed, however kw values of gray and while matter have been reported to be similar2.
Conclusion
This is the first study investigating aged-related effects of CBF, ATT and kw using DP-pCASL in a wide range of age groups. The results showed an overall trend of decreasing CBF, increasing ATT and decreasing kw with age, although there were variations across brain regions.Acknowledgements
No acknowledgement found.References
1. Farrall, A. J., & Wardlaw, J. M. (2009). Blood–brain barrier: ageing and microvascular disease–systematic review and meta-analysis. Neurobiology of aging, 30(3), 337-352.
2. Shao, X., Ma, S. J., Casey, M., D’Orazio, L., Ringman, J. M., & Wang, D. J. (2019). Mapping water exchange across the blood–brain barrier using 3D diffusion‐prepared arterial spin labeled perfusion MRI. Magnetic resonance in medicine, 81(5), 3065-3079.
3. Dai, W., Fong, T., Jones, R.N., Marcantonio, E., Schmitt, E., Inouye, S.K., Alsop, D.C., 2017. Effects of arterial transit delay on cerebral blood flow quantification using arterial spin labeling in an elderly cohort. J Magn Reson Imaging 45, 472-481.
4. Gold, B.T., Shao, X., Sudduth, T.L., Jicha, G.A., Wilcock, D.M., Seago, E.R., Wang, D.J., 2021. Water exchange rate across the blood-brain barrier is associated with CSF amyloid-beta42 and cognitive performance in healthy older adults. Alzheimer's and Dementia, (Epub online).
5. Satterthwaite, T. D., Shinohara, R. T., Wolf, D. H., Hopson, R. D., Elliott, M. A., Vandekar, S. N., ... & Gur, R. E. (2014). Impact of puberty on the evolution of cerebral perfusion during adolescence. Proceedings of the National Academy of Sciences, 111(23), 8643-8648.
Figures