1294
Multiparametric MRI, sodium MRI, and PSMA PET of prostate cancer with histological validation of Gleason grade
Josephine Tan1, Alireza Akbari1, Matthew Fox2, Mena Gaed3, Madeleine Moussa4, Jose A Gomez4, Zahra Kassam5, William Pavlosky5, Joseph L Chin6, Stephen Pautler6, Nicholas Power6, Aaron D Ward1,2, Glenn S Bauman1,2, Jonathan D Thiessen1,2,5, and Timothy J Scholl1,3,7
1Medical Biophysics, University of Western Ontario, London, ON, Canada, 2Lawson Health Research Institute, London, ON, Canada, 3Robarts Research Institute, University of Western Ontario, London, ON, Canada, 4Pathology and Laboratory Medicine, University of Western Ontario, London, ON, Canada, 5Medical Imaging, University of Western Ontario, London, ON, Canada, 6Surgery, Division of Urology, University of Western Ontario, London, ON, Canada, 7Ontario Institute for Cancer Research, Toronto, ON, Canada
1Medical Biophysics, University of Western Ontario, London, ON, Canada, 2Lawson Health Research Institute, London, ON, Canada, 3Robarts Research Institute, University of Western Ontario, London, ON, Canada, 4Pathology and Laboratory Medicine, University of Western Ontario, London, ON, Canada, 5Medical Imaging, University of Western Ontario, London, ON, Canada, 6Surgery, Division of Urology, University of Western Ontario, London, ON, Canada, 7Ontario Institute for Cancer Research, Toronto, ON, Canada
Synopsis
This abstract focuses on the development of an imaging assay based on multiparametric MRI and sodium (23Na) MRI to discriminate between low- and high-grade prostate cancer (PCa) lesions, using Gleason grade defined by whole-mount histopathology as the gold standard and PET targeting the prostate-specific membrane antigen (PSMA) as a validation. Data from the first patient (Gleason score 7) demonstrates that PSMA-PET may be superior in detecting PCa lesions in the transition zone and distant metastases while the sensitivity of the current 23Na radiofrequency system in this study is limited to lesions in the peripheral zone of the prostate.
Introduction
Prostate-specific membrane antigen (PSMA)-PET is a very sensitive molecular imaging technique that targets the PSMA protein overexpressed on most prostate cancer (PCa) cells. In combination with multiparametric MRI, which plays a growing clinical role in localizing and characterizing PCa, PSMA-PET offers very high lesion-detection specificity [1], [2]. However, limited accessibility, cost, and repeated dose of the radioactive tracer limits the utility of a combined PSMA-PET/MRI system for PCa imaging. To address these challenges, this study focuses on the development of single-modality assay based on multiparametric MRI and sodium (23Na) MRI to discriminate between low- and high-grade PCa lesions, using Gleason grade defined by whole-mount histopathology after prostatectomy as the gold standard and PSMA-PET as a validation. Tissue sodium concentration in human PCa with 23Na MRI was recently shown to significantly correlate with histological lesion grade [3]. The addition of 23Na MRI to multiparametric MRI may provide similar lesion characterization as PSMA-PET and supplement prostate biopsies conventionally used to assess PCa aggressiveness.Methods
The imaging assay was evaluated in the first patient (62 years old, 95.2 kg, Gleason score=7) from a planned cohort of 45 men with biopsy-proven PCa prior to prostatectomy using a 3 Tesla PET/MRI scanner (Siemens Biograph mMR). The radiofrequency system was PET-compatible and consisted of a custom-built 23Na RF transmit body coil (32.6 MHz), a 16-channel flexible array for 1H imaging, and a rigid dual-tuned receive endorectal surface coil for 23Na/1H imaging [4]. The MR imaging panel included 23Na MRI (TE=2.3ms, TR=218ms, FOV=20cm2, in-plane resolution=3mm2, scan time=32min), followed by T2W MRI (TE=101ms, TR=1700ms, FOV=22.6cm2, in-plane resolution=0.70mm2), dynamic contrast-enhanced (DCE) MRI (TR=5.02ms, TE=1.95ms, in-plane resolution=1.125mm2, 0.1mmol/kg Gadovist), and diffusion-weighted MRI (DWI) (TE=96ms, TR=5200ms, in-plane resolution=1.625mm2, b-values=0-800s/mm2). An apparent diffusion coefficient (ADC) map was generated by fitting DWI data with a monoexponential model. PSMA-PET (3D mode, resolution=4.17×4.17×2.03mm3, frame duration=20min) was acquired 134 minutes after the administration of 410 MBq of [18F]PSMA-1007. The boundary of the primary lesion was defined by 40% of the maximum standardized uptake value (SUVmax) [5]. After prostatectomy, 3 internal and 7 external MRI-visible fiducial markers were applied to the prostate specimens prior to ex vivo T1W and T2W MRI to facilitate co-registration of imaging data and histopathology. Whole-mount sections of the specimen were then stained with hematoxylin and eosin, digitized, and analyzed by a pathologist to produce high-resolution annotations of Gleason grade throughout the sections. Registration of the ex vivo images to digital histopathology was performed using a least-squares best-fit affine transformation algorithm [6] and permitted regional comparisons of qualitative imaging metrics with the pathologist’s determination of Gleason grade.Results and Discussion
High [18F]PSMA-1007 uptake localized the primary intraprostatic lesion in the left transition zone (SUVmax=7.76). Histopathology confirmed the presence of a Gleason grade 4 and 7 (3+4) cancer in this region. Disease was also visible outside of the prostate. The primary lesion defined by the boundary threshold of 40% SUVmax (volume=2.5 cm3) corresponded to a mildly hypointense region on the T2W image, restricted diffusion (bright signal on DWI, dark signal on the ADC map) and early, mild enhancement on DCE. From the raw 23Na MRI data, the primary lesion corresponded to a lower sodium signal relative to that observed 1cm from the endorectal coil. Based on these preliminary results, PET of [18F]PSMA-1007 may be superior in detecting PCa lesions in the transition zone and distant metastases, while, using the current MRI radiofrequency hardware, 23Na MRI may be more sensitive to lesions in the peripheral zone where majority of PCa originates. Future work includes 1) comparison of RF coil sensitivity between the current 23Na endorectal coil to an in-house built 23Na transmit/receive surface coil for prostate imaging; 2) correction of the sodium signal for RF coil sensitivity to create absolute tissue sodium concentration maps, 3) registration of the Gleason-graded whole-mount sections with the PET/MRI images using a non-rigid thin-plate spline algorithm developed in a previous study [6], and 4) statistical analysis to determine significant correlations between quantitative imaging metrics (sodium concentration from 23Na, ADC from DWI, kinetic parameters from DCE) and histological Gleason grade.Conclusions
This work shows that the sodium signal acquired by the current 23Na RF system setup is limited to the peripheral zone of the prostate, while PET of [18F]PSMA-1007 was able to localize a lesion corresponding to a Gleason grade of 3 and 3+4, as well as a metastatic lesion. Sodium signal intensity corrections and use of a 23Na transit/receive surface coil may improve the ability of a single-modality imaging assay comprised of 23Na MRI and multiparametric MRI to detect PCa lesions similar to PSMA-PET.Acknowledgements
This work is funded by the U.S. Department of Defense (PC180055) and the Ontario Institute for Cancer Research (IA-028).References
[1] G. Bauman et al., “[ 18 F]-DCFPyL Positron Emission Tomography/Magnetic Resonance Imaging for Localization of Dominant Intraprostatic Foci: First Experience,” Eur. Urol. Focus, vol. 4, no. 5, pp. 702–706, 2018.[2] F. L. Giesel et al., “Detection efficacy of 18 F-PSMA-1007 PET/CT in 251 patients with biochemical recurrence of prostate cancer after radical prostatectomy,” J. Nucl. Med., vol. 60, no. 3, pp. 362–368, 2019.
[3] N. C. Broeke et al., “Characterization of clinical human prostate cancer lesions using 3.0-T sodium MRI registered to Gleason-graded whole-mount histopathology,” J. Magn. Reson. Imaging, vol. 49, no. 5, pp. 1409–1419, 2019.
[4] A. Farag et al., “Unshielded asymmetric transmit-only and endorectal receive-only radiofrequency coil for 23Na MRI of the prostate at 3 tesla,” J. Magn. Reson. Imaging, vol. 42, no. 2, pp. 436–445, 2015.
[5] S. K. B. Spohn et al., “Comparison of Manual and Semi-Automatic [18F]PSMA-1007 PET Based Contouring Techniques for Intraprostatic Tumor Delineation in Patients With Primary Prostate Cancer and Validation With Histopathology as Standard of Reference,” Front. Oncol., vol. 10, p. 600690, 2020.
[6] E. Gibson et al., “Registration of prostate histology images to ex vivo MR images via strand-shaped fiducials,” J. Magn. Reson. Imaging, vol. 36, no. 6, pp. 1402–1412, 2012.
Figures
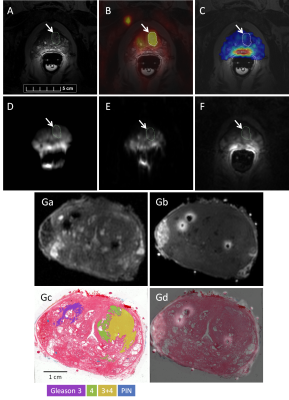
Figure 1: Representative images from (A) T2W MRI, (B) co-registered [18F]PSMA-1007 PET, (C) 23Na MRI (the raw sodium signal is averaged from 5 consecutive, six-min-long acquisitions and has been segmented to the prostate), (D) DWI (b=800s/mm2), (e) ADC map, and (F) DCE MRI. The outline (white arrow) represents the primary lesion segmentation defined by 40% SUVmax. Corresponding ex vivo (Ga) T2W and (Gb) T1W images of the prostate specimen are registered to digitized whole-mount histopathology with annotations of Gleason grade (Gc, Gd). PIN: prostatic intraepithelial neoplasia.
DOI: https://doi.org/10.58530/2022/1294