1293
Non-rigid Motion-compensated Whole-heart 18F-FDG PET and 3D T2 mapping in a hybrid PET-MR system1School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2PET Centre, St Thomas’ Hospital, King’s College London & Guys and St Thomas’ NHS Foundation Trust, London, United Kingdom, 3MR Research Collaborations, Siemens Healthcare Limited, Frimley, United Kingdom
Synopsis
Simultaneously acquired 18F-FDG PET-MR imaging and quantitative 2D T2-mapping have been suggested for improved diagnostic accuracy of cardiac sarcoidosis, however misregistration between imaging modalities makes clinical interpretation challenging. Here we used a recently proposed free-breathing motion-corrected 3D whole-heart T2-mapping sequence acquired simultaneously with 18F-FDG PET at a 3T PET-MR system. This approach enables the non-rigid motion-correction for both the 3D T2-mapping and the PET data to the same respiratory position, resulting in aligned volumes for improved clinical interpretation. In this study, we tested this approach in a patient with suspected cardiac sarcoidosis.
Introduction
Clinical guidelines for the diagnosis of cardiac sarcoidosis (CS) currently suggest a combined approach using a number of different investigations, including late gadolinium enhancement (LGE) CMR and 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) in addition to histological diagnosis [1]. Despite the excellent spatial resolution of LGE CMR for the depiction of fibrosis, it lacks a specific pattern of LGE that would be unique to CS and cannot distinguish between active disease and chronic scar. On the other hand, 18F-FDG PET can show inflammatory lesions, which can indicate active CS [2]. For optimal accuracy of a diagnosis based on 18F-FDG PET-based inflammation imaging, effective suppression of physiological FDG uptake in the cardiac muscle is key; however, patients usually fail to follow the diet required for suppression, resulting in potential false positive results [3]. Recently it has been shown that quantitative T2 mapping may enable the detection of active inflammation [4].Due to the patchy nature of CS, an efficient accelerated and non-rigid motion compensated free-breathing 3D whole-heart T2-mapping sequence is evaluated here. The framework provides myocardial inflammation characterisation throughout the whole heart and non-rigid respiratory motion fields to correct simultaneously acquired PET data in a 3T hybrid PET-MR system. This is achieved by extending a previously introduced approach for 3D translational motion-corrected T2-mapping at 1.5T [5]. The proposed sequence enables complementary and fully co-registered motion-compensated T2-mapping and 18F-FDG PET imaging. Preliminary evaluation of this PET-MR acquisition was performed in a patient with suspected cardiac sarcoidosis.
Methods
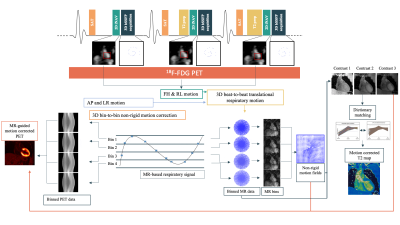
Acquisition & Reconstruction: The proposed PET-MR protocol includes an electrocardiogram (ECG) triggered free-breathing 3D whole-heart T2-mapping sequence simultaneously acquired with list-mode PET data on a 3T PET-MR system (Biograph mMR, Siemens Healthcare, Erlangen, Germany) (Figure 1). The 3D T2-mapping data are acquired using a 3-fold undersampled variable-density Cartesian trajectory [6]. The sequence includes a saturation pulse (SAT) applied at the start of each heartbeat to render the sequence heart-rate insensitive, followed by a fat saturation pulse and T2-preparation pulses of different durations (0, 28 and 55ms) immediately prior to the imaging sequence. Image navigators (iNAVs)[7] are integrated in the sequence to enable 100% respiratory scan efficiency and predictable scan time. Beat-to-beat translational motion is estimated from 2D iNAVs (foot-head and right-left directions) and virtual 3D (v3D) iNAV based on autofocus [8,9] (right-left and anterior-posterior direction).MR reconstruction: the v3D iNAV respiratory motion is used to motion-correct and bin the MR data and produce respiratory-resolved 3D MR images. 3D non-rigid motion is then estimated and incorporated into a motion-compensated reconstruction [10] with patch-based low-rank regularization [5] to produce motion-free datasets. T2-maps are computed using dictionary-matching [11].
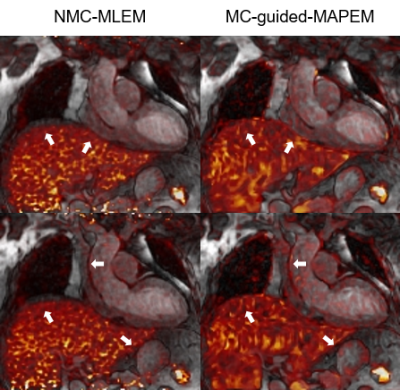
PET reconstruction: the v3D iNAV respiratory motion is used to bin the PET data into the same positions as the MR data. To improve the alignment between the μ-map and PET image position, the conventionally breath-held μ-map is registered to the last contrast of the 3D T2-mapping. This image is also used to perform MR-guided motion-corrected PET reconstruction [12], incorporating the non-rigid motion fields estimated from the MR reconstruction. This approach allows to motion-correct both the MR and PET data to the same respiratory position [13], enabling direct fusion of both datasets for analysis and interpretation.
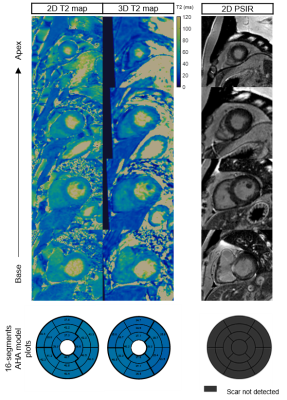
Imaging & Analysis: A male patient (58 yo) was scanned on a 3T PET-MR scanner using the proposed approach. Patient received an FDG injection of 374.4 MBq and was scanned ~2.5 hours post injection. 3D T2-mapping data was acquired during mid-diastole (acquisition window = 102ms). MR imaging parameters included: coronal orientation, FOV=336x336x156mm3, 1.5mm3 isotropic resolution, bandwidth=675Hz/px, TR/TE=3.45/1.57ms, flip angle=15°, acquisition time = 11.5min. List-mode PET data were acquired during the whole 3D T2 mapping sequence. Conventional breath-held 2D T2 maps were also acquired in short axis orientation at apical, mid-cavity and basal slices for comparison purposes (imaging parameters: resolution = 1.9x1.9x8mm3, T2-preparation pulses = 0, 28, 55 ms, flip angle=12°, bandwidth=1155 Hz/px). Conventional breath-held 2D LGE images were acquired at the same imaging locations for assessment of myocardial fibrosis/scar.
Results
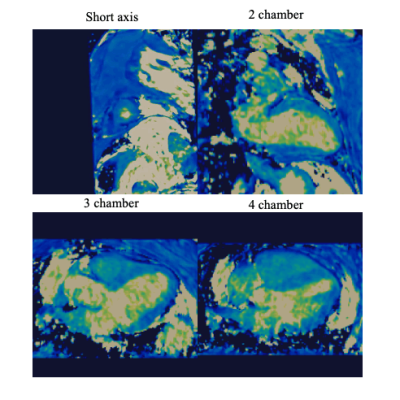
The proposed protocol was completed successfully. T2 values of the proposed 3D T2 mapping sequence were slightly underestimated compared with the conventional 2D T2 mapping (Figure 2), however only 3 slices were acquired with the conventional 2D T2 map, while 104 slices at 1.5 isotropic resolution were analysed for the 3D T2 map 16-segment AHA evaluation (Figure 3). Motion correction visually improved image quality in the PET data and improved correspondence to the 3D MR data compared to conventional uncorrected PET reconstructions (white arrows on Figure 4). There was no T2 elevation, LGE presence or 18F-FDG uptake in this subject (Figure 2).Conclusion
A novel approach for simultaneously acquired non-rigid motion corrected 18F-FDG PET and 3D T2 mapping data was successfully evaluated in a patient with suspected cardiac sarcoidosis. T2 values were slightly underestimated with the proposed approach in comparison to conventional 2D mapping, and further comparisons in patients are needed. Improved PET images due to motion correction calls for future studies in more patients with cardiac sarcoidosis undergoing simultaneous PET-MR.Acknowledgements
This work was supported by EPSRC (EP/L015226/1, EP/P032311/1, EP/P007619/1 and EP/P001009/1) and the Wellcome/EPSRC Centre for Medical Engineering (NS/A000049/1).References
[1] Birnie DH, Sauer WH, Bogun F, Cooper JM, Culver DA, Duvernoy CS, et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Hear Rhythm 2014;11:1305–23. https://doi.org/10.1016/j.hrthm.2014.03.043.
[2] Ishimaru S, Tsujino I, Takei T, Tsukamoto E, Sakaue S, Kamigaki M, et al. Focal uptake on 18F-fluoro-2-deoxyglucose positron emission tomography images indicates cardiac involvement of sarcoidosis†. Eur Heart J 2005;26:1538–43. https://doi.org/10.1093/eurheartj/ehi180.
[3] Joshi N V, Vesey AT, Williams MC, Shah AS V, Calvert PA, Craighead FHM, et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet 2014;383:705–13. https://doi.org/10.1016/S0140-6736(13)61754-7.
[4] Crouser ED, Ono C, Tran T, He X, Raman S V. Improved detection of cardiac sarcoidosis using magnetic resonance with myocardial T2 mapping. Am J Respir Crit Care Med 2014;189:109–12. https://doi.org/10.1164/rccm.201309-1668LE.
[5] Bustin A, Milotta G, Ismail TF, Neji R, Botnar RM, Prieto C, et al. Accelerated free-breathing whole-heart 3D T2 mapping with high isotropic resolution. Magn Reson Med 2019;83:988–1002. https://doi.org/10.1002/mrm.27989.
[6] Prieto C, Doneva M, Usman M, Henningsson M, Greil G, Schaeffter T, et al. Highly efficient respiratory motion compensated free-breathing coronary mra using golden-step Cartesian acquisition. J Magn Reson Imaging 2015;41:738–46. https://doi.org/10.1002/jmri.24602.
[7] Henningsson M, Koken P, Stehning C, Razavi R, Prieto C, Botnar RM. Whole-heart coronary MR angiography with 2D self-navigated image reconstruction. Magn Reson Med 2012;67:437–45. https://doi.org/10.1002/mrm.23027.
[8] Psenicny A, Cruz G, Munoz C, Hajhosseiny R, Kuestner T, Kunze KP, et al. Whole-heart CMRA non-rigid motion compensation with autofocus virtual 3D iNAV. ISMRM 2021 2021. https://cds.ismrm.org/protected/21MPresentations/abstracts/0009.html (accessed June 21, 2021).
[9] Atkinson D, Hill DLG, Stoyle PNR, Summers PE, Keevil SF. Automatic correction of motion artifacts in magnetic resonance images using an entropy focus criterion. IEEE Trans Med Imaging 1997;16:903–10. https://doi.org/10.1109/42.650886.
[10] Batchelor PG, Atkinson D, Irarrazaval P, Hill DLG, Hajnal J, Larkman D. Matrix description of general motion correction applied to multishot images. Magn Reson Med 2005;54:1273–80. https://doi.org/10.1002/mrm.20656.
[11] Weigel M. Extended phase graphs: Dephasing, RF pulses, and echoes - pure and simple. J Magn Reson Imaging 2015;41:266–95. https://doi.org/10.1002/jmri.24619.
[12] Munoz C, Ellis S, Nekolla SG, Kunze KP, Vitadello T, Neji R, et al. MR-guided motion-corrected PET image reconstruction for cardiac PET-MR *. J Nucl Med 2021. https://doi.org/10.2967/jnumed.120.254235.
[13] Munoz C, Neji R, Cruz G, Mallia A, Jeljeli S, Reader AJ, et al. Motion‐corrected simultaneous cardiac positron emission tomography and coronary MR angiography with high acquisition efficiency. Magn Reson Med 2018;79:339. https://doi.org/10.1002/mrm.26690.
Figures