1259
Determinants of MR Relaxation in the Extremely Preterm Brain at Adolescence: Myelin and Iron1Mechanical Engineering, Boston University, Boston, MA, United States, 2Boston University Medical Center, Boston, MA, United States, 3University of North Carolina at Chapel Hill, Chapel Hill, NC, United States, 4University of Massachusetts, Worcester, MA, United States
Synopsis
Purpose: To describe the dependencies of multiparametric quantitative MRI (MP-qMRI) on myelin and iron content in the extremely preterm born brain at adolescence. Methods: Algorithms for a fast exchange relaxation (FER) model and MP-qMRI create maps of R1, R2, myelin, and iron in 30 participants using MR images obtained with the triple TSE pulse sequence at age 15 years. Results: R1 and R2 have linear dependencies with myelin and iron content, respectively. Conclusion: Application of a FER model produces coregistered maps of myelin and iron content which exhibit unique influences on the relaxation of white matter and gray matter regions.
Purpose
While survival rates of children born extremely preterm (EP) (gestational age < 28 weeks) have greatly increased, they remain at an elevated risk of neurological disability due in part to perinatal infection and systemic inflammation, which may induce altered central nervous system (CNS) architecture throughout aging1. Of the microarchitectural components in the CNS, myelin and iron play prominent roles in the transmission of neural impulses and maintenance of normal physiological brain function2. They have also been observed to follow interrelated pathways, from normal aging to neurodegenerative disease3; however, many aspects of cerebral myelin and iron biology, including the influence on relaxation rates, in the white matter and gray matter require additional investigation. In this work, a fast exchange relaxation model and multiparametric quantitative MRI (MP-qMRI) were applied to the extremely preterm born brain at adolescence. Furthermore, the purpose of this study was to establish relationships between the primary qMRI relaxation parameters of tissue R1 and R2 and regional myelin and iron content.Materials and Methods
This study was approved by the Institutional Review Board of the University of North Carolina at Chapel Hill (UNC-CH), a participating institution of the Extremely Low Gestational Age Newborn – Environmental Influences on Child Health Outcomes (ELGAN-ECHO) Study. A sub-sample of 30 participants (14 females and 16 males, mean age: 15.4 ± 0.4 years) were randomly selected from the ELGAN-ECHO population imaged at a single site (UNC-CH) and were evaluated with a 3T MRI protocol using the triple turbo spin echo (TSE) pulse sequence. This triple weighting acquisition consists of concatenated long repetition time dual echo turbo spin echo (DE-TSE) and short repetition time single echo turbo spin echo (SE-TSE) sequences implemented with identical scan geometry and receiver settings. Typical imaging parameters: voxel = 0.5x0.5x2 mm3, TE1,2eff = 12 ms, 102 ms, TRlong = 10 s, TRshort = 0.5 s, with a 7:34 minutes scan time. Participant images were free of braces-induced magnetic susceptibility and severe motion artifacts. MP-qMRI algorithms for mapping the normalized proton density, R1, and R2 were programmed in Python (version 3.8.11) with the Anaconda Navigator (version 2.0.4) according to the Bloch equation solution as applicable to the triple TSE. The maps were processed to create synthetic R1 and R2 texture maps, and after applying a fast exchange relaxation model the myelin and iron content could be calculated in a voxel-wise manner. The mean R1, R2, myelin content, and iron content were calculated for 11 regions of interest (ROI) drawn in the deep gray matter4 and white matter5. Linear regression analysis was performed to assess whether a significant association existed between the MP-qMRI and respective myelin and iron content (P < 0.05).Results
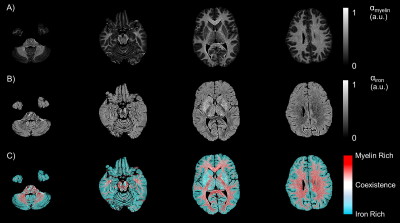
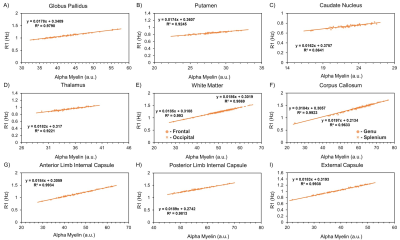
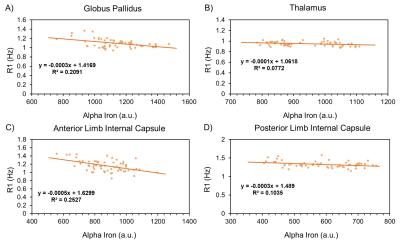
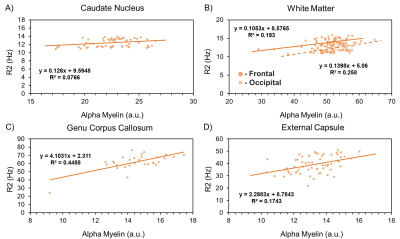
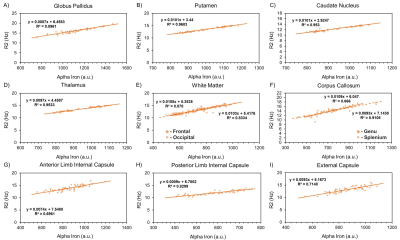
The resulting myelin and iron content maps depict expected spatial distributions of myelin and iron, with myelin content predominantly expressed in the white matter (Figure 1A), and iron content most prevalent in the deep and cortical gray matter (Figure 1B). Eleven ROIs were drawn in the globus pallidus (GP), putamen, caudate nucleus (CN), thalamus, frontal (FWM) and occipital white matter (OWM), genu (GCC) and splenium of the corpus callosum (SCC), anterior (ALIC) and posterior limbs of the internal capsule (PLIC), and the external capsule (EC). R1 was linearly associated with myelin content in all 11 ROIs (Figure 2), while only the GP (r, -0.46; P < 0.001), thalamus (r, -0.28; P = 0.03), ALIC (r, -0.50; P <0.001), and PLIC (r, -0.32; P = 0.01) were observed to have linear associations between R1 and iron content (Figure 3). R2 was linearly associated with myelin content (Figure 4) in the CN (r, 0.28; P = 0.03), FWM (r, 0.44; P < 0.001), OWM (r, 0.51; P < 0.001), GCC (r, 0.67; P < 0.001), and EC (r, 0.42; P < 0.001), but was linearly associated with iron content in all 11 ROIs (Figure 5).Discussion and Conclusions
A fast exchange relaxation model relying on MP-qMRI and synthetic MRI has been applied to the extremely preterm born brain at adolescence, providing coregistered maps of R1, R2, myelin content, and iron content. The dependence of R1 and R2 on myelin and iron content, respectively, was confirmed throughout the white and deep gray matter. The weak influence of iron accumulation as an R1 relaxer was demonstrated, while myelin content was shown to be associated with R2 of white matter regions. Further studies are needed to confirm these findings in a broader extremely preterm cohort, and to identify possible associations of regional myelin and iron content with neurocognitive outcomes.Acknowledgements
This work was supported in part by the National Institute of Neurological Disorders and Stroke (5U01NS040069-05 and 2R01NS040069-09), National Institutes of Health Office of the Director (5UH3OD023348-06), and the National Institute of Child Health and Human Development (5P30HD018655-28).References
1. O'Shea T, Allred E, Dammann O, et al. The ELGAN study of the brain and related disorders in extremely low gestational age newborns. Early human development 2009;85(11):719-725.
2. Ward RJ, Zucca FA, Duyn JH, Crichton RR, Zecca L. The role of iron in brain ageing and neurodegenerative disorders. The Lancet Neurology 2014;13(10):1045-1060.
3. Khattar N, Triebswetter C, Kiely M, et al. Investigation of the association between cerebral iron content and myelin content in normative aging using quantitative magnetic resonance neuroimaging. NeuroImage 2021:118267.
4. Langkammer C, Krebs N, Goessler W, et al. Quantitative MR imaging of brain iron: a postmortem validation study. Radiology 2010;257(2):455-462.
5. Wakana S, Jiang H, Nagae-Poetscher LM, Van Zijl PC, Mori S. Fiber tract–based atlas of human white matter anatomy. Radiology 2004;230(1):77-87.
Figures