1257
Impact of Diffusion Signal Harmonisation on Voxel-Wise Analysis of Mild Traumatic Brain Injury
Stefan Winzeck1,2, Sophie Richter2, Maira Siqueira Pinto3, Evgenios N. Kornaropoulos4, Virginia F.J. Newcombe2, Ben Glocker1, David K. Menon2, and Marta M. Correia5
1BioMedIA, Department of Computing, Imperial College London, London, United Kingdom, 2University Division of Anaesthesia, Department of Medicine, University of Cambridge, Cambridge, United Kingdom, 3Universitair Ziekenhuis Antwerpen, Antwerp, Belgium, 4Clinical Sciences, Lund, Diagnostic Radiology, Lund University, Lund, Sweden, 5MRC Cognition and Brain Sciences Unit, University of Cambridge, Cambridge, United Kingdom
1BioMedIA, Department of Computing, Imperial College London, London, United Kingdom, 2University Division of Anaesthesia, Department of Medicine, University of Cambridge, Cambridge, United Kingdom, 3Universitair Ziekenhuis Antwerpen, Antwerp, Belgium, 4Clinical Sciences, Lund, Diagnostic Radiology, Lund University, Lund, Sweden, 5MRC Cognition and Brain Sciences Unit, University of Cambridge, Cambridge, United Kingdom
Synopsis
The comparison of voxel-wise regression analysis of a multi-centre diffusion MRI study showed that similar results could be achieved regardless of whether prior data harmonisation was applied or not. However, harmonisation had a positive impact on increasing the effect size found between mild traumatic brain injury patients and controls.
Introduction
Diffusion-weighted imaging (DWI) signal is affected by the scanner used for data acquisition1,2, which makes data harmonisation a concern for multi-centre studies. Among others3,4, ComBat has been demonstrated to be successful in reducing scanner biases5. However, it also has been reported to reduce biological variability of interest when applied on a region-wise level6. We compare results from a multi-centre mild traumatic brain injury (mTBI) study before and after voxel-wise harmonisation via ComBat to see if it improves detection of white matter abnormalities.Methods
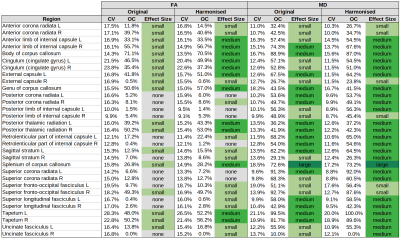
The analysis included 105 controls and 162 mTBI patients (Glasgow coma scale7 ≥ 13) scanned during the sub-acute phase (10-29 days post-injury) showing no visible intracranial pathology (Marshall score8 = 1). All subjects were collected for the CENTER-TBI study9 at ten sites on 12 scanners with similar DWI protocols (b = 0, 1000 s/mm2, ∼30 direction)10. DWI images were denoised via MPPCA11, corrected for Gibbs ringing artefacts12,13, eddy current distortions14, head motion14, and field inhomogeneities15. Fractional anisotropy (FA) and mean diffusivity (MD) maps were computed with weighted least-squares tensor fitting16 and non-linearly registered17 to the JHU-ICBM FA template (1mm)18. The quality of spatial normalisation was assessed by computing the zero-normalised cross-correlation (ZNCC)19 between warped FA maps and the template image (ZNCC [mean 土 std ] = 0.87 土 0.02). All 267 subjects were used to harmonise FA or MD maps across scanners, considering age at scan, sex and disease status as confounders20. Both original and harmonised images were smoothed (FWHM = 4 mm)16. Lastly, four generalised linear models (GLMs)21 were fitted to find divergent FA or MD between patients and controls (5000 permutations, confounders: scanner, age at scan and sex): GLM1: all original FA, GLM2: all harmonised FA, GLM3: all original MD, GLM4: all harmonised MD. Significant abnormalities (p≤ 0.05) were identified after family-wise error correction. The magnitude of deviations of mean values within 48 regions of the JHU-ICBM atlas18 between controls and patients was estimated with Cohen’s d effect size22. The impact of harmonisation was evaluated by computing coefficient of variation (CV) maps as the ratio of standard deviation and mean FA or MD from all controls at any given voxel. Mean CVs within the atlas regions were computed to summarise local variations. A region's occupancy (OC) was defined as the percentage of a region being affected by abnormal FA or MD. Total volumes (Vol) of voxels found to be significantly different between categories were computed for all regions.Results
CV: Although the mean CV values were decreased for all regions after harmonisation, the change was minimal (median [min, max]: FA: CVoriginal = 16.5% [9.9%, 28.3%], CVharmonised = 15.4% [9.1%, 26.5%]; MD: CVoriginal = 15.2% [9.6%, 28.7%], CVharmonised = 13.6% [8.7%, 26.8%]).FA: FA was significantly lower in patients compared to controls. The total volume of regions with reduced FA in patients was higher after (38.8cm3) than before (36.6cm3) harmonisation. Region occupancy was marginally increased after harmonisation. However, the same regions were affected, regardless whether data were harmonised prior to the regression analysis or not. The three most affected regions with lowered FA were the body (OCoriginal = 71.1%, OCharmonised = 70.5%) and genu (OCoriginal = 50.6%, OCharmonised = 57.7%) of the corpus callosum as well as the right anterior limb of internal capsule (OCoriginal = 55.7%, OCharmonised = 56.7%). Largest volumes of lowered FA were found in the splenium (Voloriginal = 3.4cm3, Volharmonised = 3.6cm3), genu (Voloriginal = 4.5cm3, Volharmonised = 5.1cm3) and body (Voloriginal = 9.8cm3, Volharmonised = 9.7cm3) of the corpus callosum (negligible differences between original and harmonised data). The genu (ΔVol = 0.6cm3) and the left external capsule (ΔVol = 0.5cm3) displayed most divergent results, with both volumes being larger for harmonised FA maps.
MD: MD was consistently elevated for patients compared to controls. Most impacted regions were the left (OCoriginal = 99.5%, OCharmonised = 100%) and right (OCoriginal = 81.7%, OCharmonised = 89.6%) tapetum, the left superior corona radiata (OCoriginal = 91.3%, OCharmonised = 92.0%) and the right superior fronto-occipital fasciculus (OCoriginal = 92.7%, OCharmonised = 87.6%). Largest volumes of elevated MD were located in the left superior corona radiata (Voloriginal = 6.9cm3, Volharmonised = 6.9cm3) as well as the splenium (Voloriginal = 9.2cm3, Volharmonised = 9.3cm3) and genu (Voloriginal = 12.2cm3, Volharmonised = 12.0cm3). The right anterior (ΔVol = 0.5cm3) and superior corona radiata (ΔVol = 0.6cm3) showed the largest volume differences.
Effect Size: Although abnormalities found for FA and MD were comparable before and after harmonisation, the effect size changed considerably. Twelve out of 18 regions changed from a small to a medium effect size for FA abnormalities after harmonisation. The same change was observed for eight out of 14 regions for MD abnormalities (Table 1).
Discussion & Conclusion
Voxel-wise harmonisation of diffusion parameters via ComBat only decreased variation across sites marginally, indicating that high variation is driven by more than just scanner effects, e.g. age differences. The extent and location of abnormalities were highly similar regardless of the harmonisation, demonstrating that a large enough database could be sufficient to regress out scanner effects during GLM analysis. Nonetheless, the increased effect size encourages data harmonisation prior to any statistical analysis.Acknowledgements
No acknowledgement found.References
- Zhu, Tong, et al. "Quantification of accuracy and precision of multi-center DTI measurements: a diffusion phantom and human brain study." Neuroimage 56.3 (2011): 1398-1411.
- Teipel, Stefan J., et al. "Multicenter stability of diffusion tensor imaging measures: a European clinical and physical phantom study." Psychiatry Research: Neuroimaging 194.3 (2011): 363-371.
- Tax, Chantal MW, et al. "Cross-scanner and cross-protocol diffusion MRI data harmonisation: A benchmark database and evaluation of algorithms." NeuroImage 195 (2019): 285-299.
- Pinto, Maíra Siqueira, et al. "Harmonization of Brain Diffusion MRI: Concepts and Methods." Frontiers in Neuroscience 14 (2020).
- Fortin, Jean-Philippe, et al. "Harmonization of multi-site diffusion tensor imaging data." Neuroimage 161 (2017): 149-170.
- Cetin-Karayumak, Suheyla, et al. "Exploring the limits of ComBat method for multi-site diffusion MRI harmonization." bioRxiv (2020).
- Teasdale, Graham, and Bryan Jennett. "Assessment of coma and impaired consciousness: a practical scale." The Lancet 304.7872 (1974): 81-84.
- Marshall, Lawrence F., et al. "A new classification of head injury based on computerized tomography." Journal of neurosurgery 75.Supplement (1991): S14-S20.
- Maas, Andrew IR, et al. "Collaborative European NeuroTrauma effectiveness research in traumatic brain injury (CENTER-TBI) a prospective longitudinal observational study." Neurosurgery 76.1 (2015): 67-80.
- https://www.center-tbi.eu/project/mri-study-protocols
- Veraart, Jelle, Els Fieremans, and Dmitry S. Novikov. "Diffusion MRI noise mapping using random matrix theory." Magnetic resonance in medicine 76.5 (2016): 1582-1593.
- Kellner, Elias, et al. "Gibbs‐ringing artifact removal based on local subvoxel‐shifts." Magnetic resonance in medicine 76.5 (2016): 1574-1581.
- Neto Henriques, Rafael. Advanced Methods for Diffusion MRI Data Analysis and their Application to the Healthy Ageing Brain. Diss. University of Cambridge, 2018.
- Andersson, Jesper LR, and Stamatios N. Sotiropoulos. "An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging." Neuroimage 125 (2016): 1063-1078.
- Jeurissen, Ben, et al. "Multi-tissue constrained spherical deconvolution for improved analysis of multi-shell diffusion MRI data." NeuroImage 103 (2014): 411-426.
- Smith, Stephen M., et al. "Advances in functional and structural MR image analysis and implementation as FSL." Neuroimage 23 (2004): S208-S219.
- Avants, Brian B., Nick Tustison, and Gang Song. "Advanced normalization tools (ANTS)." Insight j 2.365 (2009): 1-35.
- Mori, Susumu, et al. MRI atlas of human white matter. Elsevier, 2005.
- https://en.wikipedia.org/wiki/Cross-correlation
- https://github.com/Jfortin1/ComBatHarmonization
- Winkler, Anderson M., et al. "Permutation inference for the general linear model." Neuroimage 92 (2014): 381-397.
- Cohen, Jacob. "A power primer." Psychological bulletin 112.1 (1992): 155.
DOI: https://doi.org/10.58530/2022/1257