1251
Resolution-dependency of arterial and venous density estimates and vessel distance maps in deep gray matter1Biomedical Magnetic Resonance, Otto von Guericke University, Magdeburg, Germany, 2German Center for Neurodegenerative Disease, Magdeburg, Germany, 3Center for Behavioral Brain Sciences, Magdeburg, Germany, 4Leibniz Institute for Neurobiology, Magdeburg, Germany
Synopsis
The detection of vessel in MRI depends on the imaging resolution used. Hence, subsequent quantification with vessel densities and vessel distance mapping (VDM) is resolution dependent. In this study, the resolution dependency of both metrics was evaluated for arteries and veins in deep gray matter regions by retrospective downsampling of high-resolution vessel images. Quantitative comparison shows that with decreasing resolution the estimated vessel distances increase, while vessel densities decline. The resolution dependency of vessel densities and distances can be modeled linearly. The here found scaling factors may improve inter-study comparability of vessel densities and VDM.
Introduction
Recently, vessel distance mapping (VDM) has been introduced as a complementary tool to vessel densities to quantify the vasculature1-3. Since VDM and vessel densities are based on vessel segmentation, they correlate with each other to some extent and share the bias of being limited to detected vessels1-3. In this study, the resolution dependency of both metrics was evaluated for arteries and veins in deep gray matter regions by retrospective downsampling of high-resolution vessel images. Comparison across resolutions and studies is enabled by linear scaling factors for VDM and vessel densities.Methods
Data acquisition and processing:Five subjects were scanned at 7T (Siemens Healthineers, Erlangen, Germany) with a 32-channel head coil (Nova Medical, Wilmington, USA) after given written consent (study approved by local ethic committee). Time-of-Flight (ToF) angiography at 0.2mm isotropic resolution and susceptibility-weighted gradient echo data at 0.35mm isotropic resolution were acquired. ToF images were bias-field corrected with the N4 algorithm4. Quantitative susceptibility maps (QSM) were generated from the gradient echo data with QSMbox5. To allow segmentation of deep gray matter structures, the data was non-linearly registered to MNI space using ANTs6. Afterwards, the inverse transformation was applied to register the Harvard-Oxford subcortical atlas7 into individual subject space.
Arterial and venous densities and vessel distance mapping:
To isolate the vasculature, arteries and veins were enhanced using the Jerman Filter8, respectively. Subsequently, hysteresis thresholding (thresholds obtained by 3-class Otsu method) was used to generate vessel segmentations9. To interpolate the spares vessel information and provide vessel distance maps, the Euclidean distance of each voxel to its closest segmented vessel was computed10. Vessel densities were computed per ROI as the ratio of detected vessel volume to total ROI volume.
Assessment of the resolution dependency:
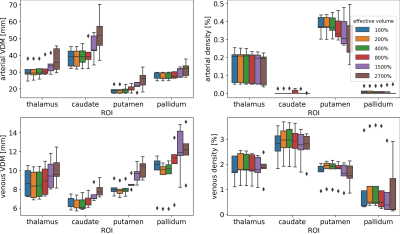
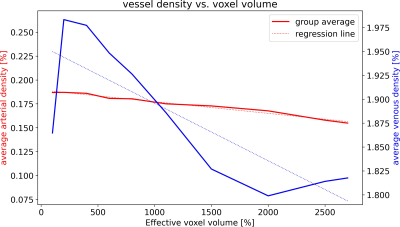
To assess the effect of the effective resolution, the data was downsampled after acquisition and prior to vessel segmentation and quantification. To that end, the data was transferred to k-space and the high frequency components were set to zero. Thus, the nominal voxel size remained constant throughout the analysis, but the effective resolution decreased, hence, the effective voxel length and volume increased. Retrospective downsampling was performed to increase the effective voxel volume from 100% to 200%, 400%, 600%, 800%, 1500%, 2500% and 2700%, respectively. Due to the cubic relation of voxel length and volume, this corresponds to a factor 1.0 to 3.0 increase of the effective voxel edge length. Hence, for each subject, arterial and venous densities as well as VDM were computed for 8 different effective resolutions. Box plots were used to compare the mean densities and distances of all subjects bilaterally in the thalamus, putamen, pallidum, and caudate nucleus across the different effective resolutions. To investigate the resolution dependency throughout deep gray matter, the vessel densities and distances across all regions and subjects were averaged and plotted against the effective voxel volume. Linear regression, correlation coefficients, and root-mean-square-percent-error (RMSPE) were used to investigate how accurately the resolution dependency can be modeled by a linear scaling factor.
Results
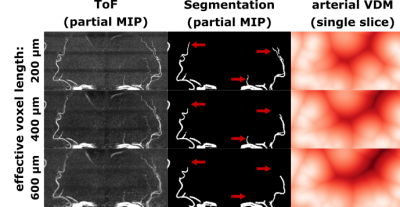
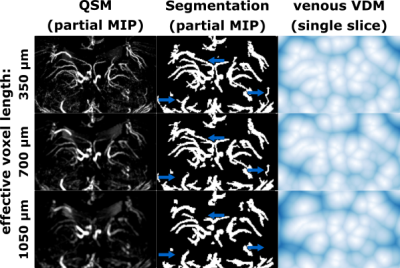
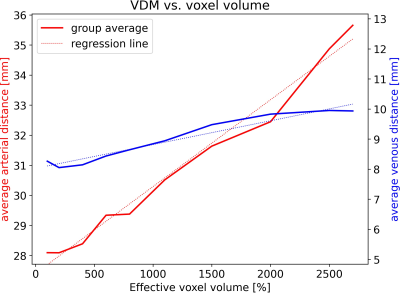
With increasing effective voxel length, small vessels get blurred, preventing eventually their segmentation (see Fig.1&2). In line with these visual impressions, the quantitative region-specific comparison (see Fig.3) shows that with increasing effective voxel length the estimated vessel distances increase as well, while vessel densities decline. Note that downsampling can reduce the noise level and, therefore, improve vessel segmentation. For venous density estimates, the downsampling-induced noise suppression, increased venous density (i.e. 100% to 200%). Regardless, from 200% a steady decline in densities can be observed. The resolution dependency of vessel densities and distances can be modeled linearly across deep gray matter structures (see Fig.4&5): correlation coefficient and RMSPE 0.99 and 1.08% for arterial VDM, 0.97 and 0.178% for venous VDM, -0.99 and 0.84% for arterial density, and -0.81 and 2.10% for venous density, respectively. The estimates’ slope and intercepts (0.003 & 27.41mm, 0.001 & 8.03mm, 0.0001 & 0.19%, 0.00006 & 1.96% for arterial VDM, venous VDM, arterial density, and venous density, respectively) enable scaling of the results across resolutions to allow inter-study comparability.Discussion
Quantification of arterial and venous vasculature in deep gray matter regions with vessel densities and VDM depends on the imaging resolution used. By retrospectively reducing the effective resolution, the vessel density estimates decreased while vessel distances increased. For downsampling, k-space zero-filling instead of k-space cropping was used to keep the nominal resolution constant. Hence, the discretization of VDM (i.e. voxel length) was constant and no potential bias due to downsampling the ROI masks was introduced. Albeit downsampling can reduce initially the image noise and, therefore, improved vessel segmentation, the resolution dependency of vessel densities and VDM can be approximated reasonably well as linear. VDM was less sensitives to downsampling-induced denoising and showed a higher linear correlation to the effective voxel volume, arguably rendering it more robust for inter-study comparisons. After verification in a larger cohort and in presence of (vascular) pathologies, the proposed scaling factors may allow comparison of vessel estimates independent of the imaging resolution used.Conclusion
To improve reproducibility and comparability, we recommend to report vessel densities and VDM estimates always w.r.t. the (effective) voxel volume used.Acknowledgements
This work was funded by the DFG-MA 9235/1-1References
1. Mattern H, Speck O. Vessel distance mapping. 36th Annual Scientific Meeting of European Society for Magnetic Resonance in Medicine and Biology.
2. Mattern H, Schreiber S, Speck S. Vessel distance mapping for deep gray matter structures. 29th Annual Meeting of International Society of Magnetic Resonance in Medicine.
3. Mattern H. Vessel distance mapping of the aging subcortical venous vasculature.37th Annual Scientific Meeting of European Society for Magnetic Resonance in Medicine and Biology.
4. Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA, Gee JC. N4ITK: improved N3 bias correction. IEEE Trans Med Imaging. 2010;29(6):1310–1320 doi: 10.1109/TMI.2010.2046908 .
5. Acosta-Cabronero J, Milovic C, Mattern H, Tejos C, Speck O, Callaghan MF. A robust multi-scale approach to quantitative susceptibility mapping. Neuroimage. 2018. doi: 10.1016/j.neuroimage.2018.07.065 .
6. Avants B, Epstein C, Grossman M, Gee J. Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Medical Image Analysis. 2008;12(1):26–41 doi: 10.1016/j.media.2007.06.004 .
7. Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006 Jul 1;31(3):968-80.
8. Jerman T, Pernus F, Likar B, Spiclin Z. Enhancement of Vascular Structures in 3D and 2D Angiographic Images. IEEE Transactions on Medical Imaging. 2016;35(9):2107–2118. doi: 10.1109/TMI.2016.2550102 .
9. Mattern H. Openly available sMall vEsseL sEgmenTaTion pipelinE (OMELETTE). 29th Annual Meeting of International Society of Magnetic Resonance in Medicine
10. Maurer CR, Qi R, Raghavan V. A linear time algorithm for computing exact Euclidean distance transforms of binary images in arbitrary dimensions. IEEE Trans. Pattern Anal. Mach. Intell. . 2003;25(2):265–270 doi: 10.1109/TPAMI.2003.1177156 .
Figures