1249
Investigation of myelin using T1/T2 ratio imaging across multiple datasets: comparable or not?1Queen Square Institute of Neurology, University College London, London, United Kingdom, 2Neuroradiological Academic Unit, Queen Square Institute of Neurology, University College London, London, United Kingdom, 3Department of Radiology, Brest Regional Clinical Hospital, Brest, Belarus, 4Centre for Medical Image Computing, Department of Computer Science, University College London, London, United Kingdom, 5Dementia Research Centre, Queen Square Institute of Neurology, University College London, London, United Kingdom, 6Centre for Microscopy, Characterisation, and Analysis, The University of Western Australia, Perth, Australia
Synopsis
Cortical T1/T2 ratios (T1/T2r) have been used to assess myelination along the lifespan and investigate demyelination in neurological diseases. Creating a comprehensive normative population from open-access datasets could facilitate widespread adoption of this technique. Here we investigate comparability between two such publicly available datasets, OASIS3 and CamCAN. We find that they vary in terms of offset, lifespan trajectory, and presence (OASIS3) or absence (CamCAN) of hemispheric asymmetry. This variability exists despite both studies use the same scanner model, and is likely increased across different scanner models and vendors. These findings indicate that combining data from different studies requires advanced normalisation.
Introduction
Myelin is of critical importance in healthy neuronal functioning, and huge efforts have been invested to improve the ability to image myelin non-invasively. The use of T1/T2-ratio (T1/T2r) imaging enabled acquisition of myelin-sensitive maps using routine clinical MRI sequences1. This method has been used to investigate myelin development over the lifespan2,3, and examine myelin abnormalities in diseases such as multiple sclerosis4 and Alzheimer’s disease5,6. Studies using T1/T2r for myelin imaging have so far been limited to single-site or single dataset research. To allow widespread adoption of this methodology, comparability across datasets is crucial to provide normative datasets – as was recently done for brain volumetry7. In this work, we consider T1/T2r in two publicly available datasets.Methods
Data: We used data from the OASIS38 and CamCAN9 datasets. For both datasets, we included only data from 3T Siemens Skyra scanners; scans were 3D-T1-MPRAGE and 3D-T2-SPACE, both at 1 mm isotropic resolution (see Table 1 for key sequence parameter differences). Only healthy subjects were included in the analysis, which resulted in 1395 scan sessions in 902 participants (405M/497F) from OASIS3 and 653 scan sessions in 653 participants (323M/330F) from CamCAN – see Figure 1 for age distributions and numbers of visits for OASIS3.Processing
T1-weighted MRI scans were segmented using CAT12 (http://dbm.neuro.uni-jena.de/cat/) in SPM12 (https://www.fil.ion.ucl.ac.uk/spm/) into white matter (WM), grey matter (GM), and cerebrospinal fluid (CSF) probability maps, with a binary GM mask defined as those voxels where GM had the highest tissue probability. Visual quality control was performed on the segmentations. T1/T2r maps were created using MRtool10 in SPM12, and values were extracted for the left and right entire cortical hemispheres.
Analysis
Cross-sectional analysis of T1/T2r over the lifespan (using only one time) was investigated using linear, quadratic, and sigmoidal fitting for each of the datasets, using age (A; continuous), gender (G; M=1, F=0), and/or hemisphere (H; L=0, R=1) as covariates. To investigate T1/T2r trajectories across the two datasets, fits were also performed in the age-range common to both datasets (42-89y). Model fits per datasets were evaluated based on the Akaike Information Criterion (AIC), and across datasets with mean square error (MSE). For longitudinal analysis, a linear mixed-effects model was used, including a random effect of participant. This included n=338 OASIS3 participants who had more than one neuroimaging assessment.
Results
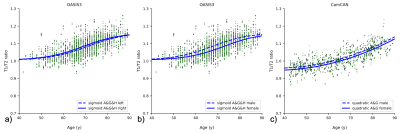
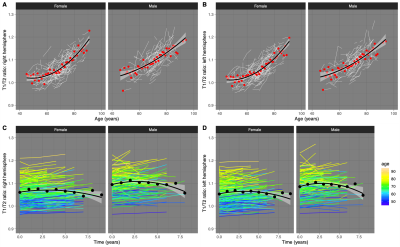
Trajectories of T1/T2r for the two datasets showed different profiles over time (Fig. 2). There are clear differences between CamCAN and OASIS3, visible across the original age ranges but, more importantly, when investigating the common age range. Not only was there a systematic offset between datasets (T1/T2r at age=40: OASIS3=1.028; CamCAN=0.954), but for OASIS3 there was a significant hemispheric asymmetry that was not present in the CamCAN dataset. Most importantly, the trajectories as age increases were significantly different too, with the sigmoidal model with age, gender, and side being the best fit for OASIS3 whereas a quadratic model with age and gender was best for CamCAN (Fig. 3). Longitudinal analysis also showed increased T1/T2r with age, with significant effects of age and age2 for both hemispheres (Figure 4A, 4B). Within-subject, neither time nor time2 were significant when accounting for age, with trajectories of T1/T2r relatively stable (Figure 4C, 4D).Discussion
Despite being scanned on the same scanner model, same vendor, using the same sequence types, with data processed with the same methods, there are significant differences between T1/T2r values as a proxy for myelin from two publicly available datasets. This may be caused by different acquisition parameters giving slightly different T1- and/or T2-weighting (Table 1), resulting in a differential influence of myelin and others factors (such as iron). Sequence implementations frequently vary between vendors, possibly resulting even larger differences in tissue weightings and further complicated across-scanner comparisons. The results obtained in this study indicate that multi-centre analysis of T1/T2r may require dedicated acquisition harmonisation, post-acquisition normalisation, or both. Ageing showed clear increases in T1/T2r in males and females in cross-sectional and longitudinal models. Individual trajectories were relatively stable when accounting for ageing effects, though a likely survivor basis whereby older participants had fewer longitudinal observations. Our results are in contrast to previous investigations of T1/T2r across the lifespan, where Grydeland et al., found T1/T2r values reaching a peak around 60 years of age before decreasing2,3. Differences could be because of a different field strength or differences in where along the GM ribbon data was sampled.Acknowledgements
Acknowledgements Data were provided in part by OASIS: OASIS-3: Principal Investigators: T. Benzinger, D. Marcus, J. Morris; NIH P50 AG00561, P30 NS09857781, P01 AG026276, P01 AG003991, R01 AG043434, UL1 TR000448, R01 EB009352. AV-45 doses were provided by Avid Radiopharmaceuticals, a wholly owned subsidiary of Eli Lilly. Data collection and sharing for this project was provided by the Cambridge Centre for Ageing and Neuroscience (CamCAN). CamCAN funding was provided by the UK Biotechnology and Biological Sciences Research Council (grant number BB/H008217/1), together with support from the UK Medical Research Council and University of Cambridge, UK.References
1. Glasser and Van Essen. Mapping human cortical areas in vivo based on myelin content as revealed by T1- and T2-weighted MRI. J Neurosci, 2011;31(32):11597-11616
2. Grydeland, Walhovd, Tamnes, et al. Intracortical myelin links with performance variability across the human lifespan: results from T1- and T2-weighted MRI myelin mapping and diffusion tensor imaging. J Neurosci, 2013;33(47):18618-18630
3. Grydeland, Vertes, Vasa, et al. Waves of maturation and senescence in microstructural MRI markers of human cortical myelination over the lifespan. Cerebral Cortex, 2019;29:1369-1381.
4. Nakamura, Chen, Ontaneda, et al. T1-/T2-weighted ratio differs in demyelinated cortex in Multiple Sclerosis. Ann Neurol, 2017;82:635-639
5. Luo, Li, Zeng, et al. Application of T1-/T2-weighted ratio mapping to elucidate intracortical demyelination process in the Alzheimer’s disease continuum. Frontiers in Neuroscience, 2019;13(904):1-13
6. Pelkmans, Dicks, Barkhof, et al. Gray matter T1-w/T2-w ratios are higher in Alzheimer’s disease. Hum Brain Mapp, 2019;40:3900-3909
7. Vinke, Huizinga, Bergtholdt, et al. Normative brain volumetry derived from different reference populations: impact on single-subject diagnostic assessment in dementia. Neurobiology of Aging, 2019;84:9-16
8. La Montagne, Benzinger,Morris, et al. OASIS-3: Longitudinal neuroimaging, clinical, and cognitive dataset for normal aging and Alzheimer’s disease. medRxiv 2019.12.13.19014902
9. Taylor, Williams, Cusack, et al. The Cambridge Centre for Ageing and Neuroscience (Cam-CAN) data repository: structural and functional MRI, MEG, and cognitive data from a cross-sectional adult lifespan sample. NeuroImage, 2017;144:262-269
10. Ganzetti, Wenderoth, and Mantini. Whole brain myelin mapping using T1- and T2-weighted MR imaging data. Frontiers in Human Neuroscience, 2014;8(671):1-14
Figures
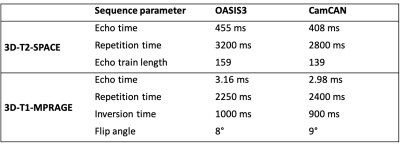
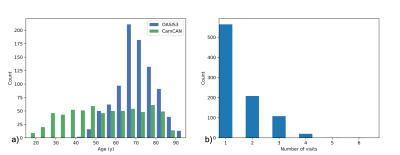
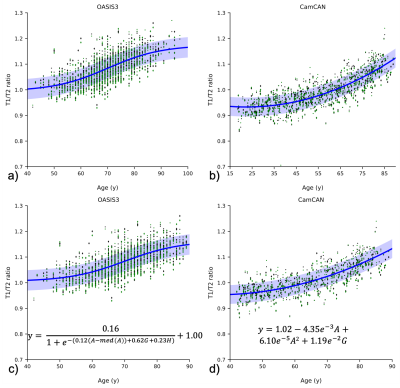
Figure 2: T1/T2 ratios for OASIS3 (a&c) and CamCAN (b&d). a) and b) show the datasets in their respective age ranges, with all model fits. c) and d) show the plots in the age range where they overlap, showing the best model fit. The blue shaded areas indicate one standard deviation above and below the best fit. Abbreviations: A=age, G=gender, H=hemisphere. Green markers indicate left hemisphere, black indicates right; dots indicate males, diamonds indicate females.