1245
Meningiomas with NF2-LossExhibit Strong Radiomics Correlations
on Contrast Enhanced T1-Weighted MRI at 3T
1Institute of Biomedical Engineering, Bogazici University, İstabul, Turkey, 2Health Institutes of Turkey, İstanbul, Turkey, 3Department of Medical Pathology, Acibadem Mehmet Ali Aydinlar University, İstanbul, Turkey, 4Department of Medical Engineering, Acibadem Mehmet Ali Aydinlar University, İstanbul, Turkey, 5Department of Neurosurgery, Acibadem Mehmet Ali Aydinlar University, İstanbul, Turkey, 6Department of Radiology, Acibadem Mehmet Ali Aydinlar University, İstanbul, Turkey
Synopsis
Meningioma is the most common primary intracranial tumor in adults, and loss of NF2 function in meningiomas has been associated with a more aggressive biology, shorter time to recurrence and shorter overall survival. In this work, radiomics based biomarkers of NF2 copy number loss (NF2-L) have been assessed. Lower grey-level co-occurrence matrix cluster shade with wavelet high-high-low pass filter, lower original image first order minimum, and higher first order skewness with wavelet low-low-high pass filter were observed in tumors with NF2-L compared to tumors with no copy number loss. The classification accuracy of NF2 molecular subsets was 0.80±0.03 (precision=0.85±0.04, recall=0.73±0.05).
Introduction
IntroductionMeningioma is the most common primary tumor of the central nervous system, which are heterogenous in their age of presentation, anatomical location as well as molecular genetic alterations.1 The majority of meningiomas are classified as benign (grade I) according to World Health Organization (WHO) 2016 grading system, while atypical (WHO grade II) or anaplastic (WHO grade III) meningiomas have shown significantly worse prognosis.2 In recent years, genetic biomarkers have contributed to the understanding of biological behavior, treatment response, and consequently prognosis of brain tumors.3 Loss of neurofibromatosis 2 (NF2) function is observed in 2/3 of all meningiomas.4 This most commonly presents as the loss of an allele of the gene (copy number loss). Additional alterations such as mutations are generally seen in addition to the copy number loss. Tumors carrying NF2 alterations have been associated with a more aggressive biology (being more invasive, prone to present in and progress to higher histological grades due to genomic instability), shorter time to recurrence and shorter overall survival.5,6 Thus, identifying the molecular subset with NF2 loss (NF2-L) at the time of diagnosis may support decision making for the treatment strategy. Radiomics is a comprehensive and quantitative method that converts medical images into “minable” high-dimensional data.7 In this study, we aim to predict the NF2-L in meningioma patients by using quantitative radiomic features obtained from preoperative contrast-enhanced (CE) T1-weighted (T1-w) MRI.
Methods
Eighty-five meningioma patients were included in this study after written informed consent (mean age=53.11±13.54 years, median age of 54 [range 26-86] years, female/male ratio:1.83). Forty patients were grade I, 42 patients were grade II, and 3 patients were grade III. The patients were scanned at pre-surgery on a 3T clinical MR scanner (Siemens Healthcare, Erlangen, Germany) using a brain tumor imaging protocol that included post-contrast (gadolinium (Gd)) T1-w SE MRI (TR/TE= 415/10 ms). Based on the assessment of surgical specimen, 38 of the tumors were NF2-L and 47 of them had no copy number loss (NF2-NL), as determined by digital droplet PCR using pre-validated Taq-man probes. The radiomics workflow of the study is given in Fig. 1. First, the CE tumor volumes were manually segmented using 3D Slicer software.8 Then, a 100 gray level normalization scale was applied to MR images. To minimize the effect of inhomogeneity between acquisitions, images were z-score normalized and resampled to the same resolution (1×1×1 mm3). A total of 1132 radiomic features were computed using PyRadiomics.9 For feature selection, collinear features were eliminated using pairwise Spearman’s correlation filter (r>±0.7) and then the features with low variance were removed from the feature set. Then, the least absolute shrinkage and selection operator (LASSO) feature selection method was applied to the training set to search the optimal predictive features. This process was repeated 100 times with different seed values and the final feature subset was determined with features with a frequency of above 80%. The differences of selected radiomics features between NF2-L and NF2-NL meningiomas were assessed by a Mann-Whitney U test. P< 0.0083 was determined as the significance level after a Bonferroni correction. Finally, NF2-L and NF2-NL meningiomas were classified using a logistic regression classifier with 10-fold cross-validation and 100 repetitions with different seed values.Results
The pairwise Spearman’s correlation and variance thresholding retained 24 predictor features, while LASSO resulted in six features for the prediction of NF2-L (frequency above 80%) (Fig. 3). Among selected radiomics features, NF2-L tumors showed significantly lower grey level co-occurrence matrix cluster shade with wavelet high-high-low pass filtering, original image first order minimum, and higher first order skewness with wavelet low-low-high pass filtering compared to NF2-NL tumors (P< 0.0083) (Fig. 2). The best performance for prediction of NF2 status was obtained with a logistic regression classifier that resulted in an accuracy of 0.80±0.03 (0.85±0.04 precision and 0.73±0.05 recall).Discussion
In the present study, we found that CE T1-w radiomic models might be helpful for the prediction of the meningioma subset with NF2-L. Previous studies reported that radiomics had the potential to estimate tumor grades and histological subtypes of meningiomas.10 Since radiomics analysis extracts a large number of predictive features from imaging data, a well-functioning feature selection process was necessary.11 In our case, LASSO selected six important radiomic features while minimizing diversity over 100 repetitions which enabled generalization of the feature selection process. Malignancy in tumors has been associated with intra-tumoral spatial heterogeneity, which is usually captured by texture and intensity-based radiomic features.12 Therefore, radiomic features have the potential to quantify the characteristics of the tumor microenvironment that are imperceptible to the human eye.Conclusion
Current findings indicate that the meningioma molecular subset with NF2-L has strong radiomics correlates on CE T1w MRI as can be determined by machine learning.Acknowledgements
This study was supported by the Scientific and Technological Research Council of Turkey
(TUBITAK) grant 119S520.
References
1. American Brain Tumor Association. Meningiomas. Published 2021. https://www.abta.org/tumor_types/meningioma/
2. Wang YC, Chuang CC, Wei KC, et al. Long Term Surgical Outcome and Prognostic Factors of Atypical and Malignant Meningiomas. Sci Rep. 2016;6(July):1-8. doi:10.1038/srep35743
3. Yaylim I, Azam S, Farooqi AA, Küçükhüseyin Ö, Ismail M, Kafadar AM. Critical Molecular and Genetic Markers in Primary Brain Tumors with Their Clinical Importance. Neurooncology - Newer Dev. Published online 2016. doi:10.5772/63550
4. Youngblood MW, Miyagishima DF, Jin L, et al. Associations of meningioma molecular subgroup and tumor recurrence. Neuro Oncol. 2021;23(5):783-794. doi:10.1093/neuonc/noaa226
5. Goutagny S, Kalamarides M. Meningiomas and neurofibromatosis. J Neurooncol. 2010;99(3):341-347. doi:10.1007/s11060-010-0339-x
6. Aboukais R, Zairi F, Baroncini M, et al. Intracranial meningiomas and neurofibromatosis type 2. Acta Neurochir (Wien). 2013;155(6):997-1001; discussion 1001. doi:10.1007/s00701-013-1692-2
7. Lambin P, Leijenaar RTH, Deist TM, et al. Radiomics: The bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol. 2017;14(12):749-762. doi:10.1038/nrclinonc.2017.141
8. 3D Slicer image computing platform. https://www.slicer.org/
9. van Griethuysen JJMM, Fedorov A, Parmar C, et al. Computational radiomics system to decode the radiographic phenotype. Cancer Res. 2017;77(21):e104-e107. doi:10.1158/0008-5472.CAN-17-0339
10. Park YW, Oh J, You SC, et al. Radiomics and machine learning may accurately predict the grade and histological subtype in meningiomas using conventional and diffusion tensor imaging. Eur Radiol. 2019;29(8):4068-4076. doi:10.1007/s00330-018-5830-3
11. Delzell DAP, Magnuson S, Peter T, Smith M, Smith BJ. Machine Learning and Feature Selection Methods for Disease Classification With Application to Lung Cancer Screening Image Data. Front Oncol. 2019;9(December):1-8. doi:10.3389/fonc.2019.01393
12. O’Connor JPB, Rose CJ, Waterton JC, Carano RAD, Parker GJM, Jackson A. Imaging intratumor heterogeneity: Role in therapy response, resistance, and clinical outcome. Clin Cancer Res. 2015;21(2):249-257. doi:10.1158/1078-0432.CCR-14-0990
Figures
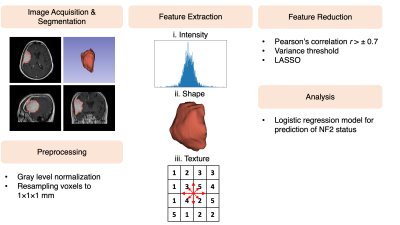
Fig. 1. The workflow of the study for prediction of NF2 status in meningioma patients.
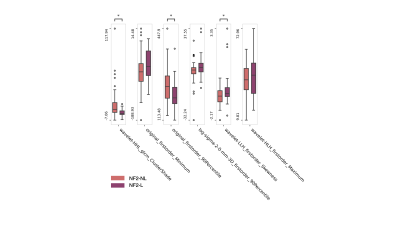
Fig. 2. The most informative features (LASSO selection frequency >80%) for differentiating NF2-NL and NF2-L meningiomas. (*P: 0.008)

Fig. 3. The selected features with frequencies of above 20% using LASSO feature selection process.