1243
Effect of radiation dose on the recovery of white matter integrity in children treated for medulloblastoma1Diagnostic Imaging, St. Jude Children's Research Hospital, Memphis, TN, United States, 2Biostatistics, St. Jude Children's Research Hospital, Memphis, TN, United States, 3Oncology, St. Jude Children's Research Hospital, Memphis, TN, United States, 4Radiation Oncology, St. Jude Children's Research Hospital, Memphis, TN, United States
Synopsis
This study reports on the effects of pediatric medulloblastoma therapy in a population of 105 subjects. Longitudinal white matter microstructure was studied starting with the post radiation examination until approximately two years after diagnosis. The tract-based spatial statistics (TBSS) pipeline was used to combine fractional anisotropy (FA) with individual dosimetry maps before linear mixed effects models were calculated for each voxel. Significant voxels were found for increasing FA over time and a decrease of FA over time associated with higher radiation dose. These results are consistent with a recovery in FA that is attenuated by higher doses of radiation.
PURPOSE
Five-year survival for medulloblastoma, the most common pediatric brain tumor, remains at approximately 80% for standard risk and 60% for high risk subjects.1 White matter (WM) microstructural damage after surgery, but before therapy has previously been reported.2 Normal appearing WM volume was similar to healthy controls at baseline but decreased after therapy. Additionally, relationships between therapy and neurocognitive decline have also been reported.3 In this work, we explored longitudinal WM microstructure changes from post irradiation until approximately two years after diagnosis and examined the relationship with radiation dose.PATIENTS AND METHODS
Subjects were collected from a single institution trial for newly diagnosed medulloblastoma (NCT01878617). Treatment included maximal surgical resection, risk-adapted craniospinal irradiation, and adjuvant chemotherapy stratified by a combination of molecular and clinical factors. Patients were imaged at five time points: baseline after surgery, after radiation therapy, immediately after chemotherapy, three months after chemotherapy, and nine months after chemotherapy. To be eligible, subjects had to have both the baseline and post radiation MRI examinations as well as dosimetry plans available. In total, 105 patients (3.3-22.8 years at diagnosis; median=9.1 years; males/females 69/36) met these criteria. Of these subjects, four were the wingless (WNT) molecular phenotype, 24 Sonic hedgehog (SHH), and 77 Non-WNT Non-SHH. Imaging and treatment protocols were approved by the local Institutional Review Board, and written informed consent was obtained from the patient, parent, or guardian, as appropriate.Diffusion tensor imaging (DTI) was collected on all subjects using a 3.0T whole-body system (Siemens Medical Systems, Iselin, NJ). Patients had either a 30 direction, two average acquisition [TR/TE=14000/120ms, b=700s/mm2, FOV=230, 1.8x1.8x2mm resolution], or a 64 direction, two reverse blip acquisition [TR/TE=4000/77.4ms, b=1500s/mm2, FOV=230, MB=4, 1.8x1.8x1.8mm resolution] collected based on the time of the examination. Parametric fractional anisotropy (FA) maps were combined from eigenvalues calculated by tensor parameters for each voxel using FMRIB’s Diffusion Toolbox4,5. Radiation dosimetry maps were generated from therapy plans and registered to the DTI (Figure 1).
Voxel-wise statistical analysis of the FA data with the corresponding individualized dosimetry maps were carried out using tract-based spatial statistics (TBSS).6 TBSS projects all subjects' FA onto a mean FA tract skeleton, before applying voxel-wise cross-subject statistics. After mapping the FA and dosimetry data onto the skeleton, a linear mixed effects model was created for each skeleton voxel from post-radiation to the end of imaging to evaluate the longitudinal evolution of the FA after irradiation while adjusting for baseline FA values. The model was written as:
Yij = β0 + β1 BYi + β2 Dosei + β4 Bagei + (β3 + β5 Dosei +β6 Bagei) tij + αi + εij
where Y is the FA at a specific voxel, BY is the FA value at the baseline examination, Dose is the dose of the patient per pixel, Bage is the baseline age, and t is the time since the post radiation timepoint. Hypotheses were tested using a likelihood ratio test with Sattherthwaite’s approximation of degrees of freedom.7 After correcting for multiple testing,8 p<=0.05 was considered significant. The results were examined against the JHU-ICBM-DTI-81 WM Atlas9 to identify the location of the significant voxels.
RESULTS
The TBSS analysis in this study was based on a total of 451 examinations and produced a skeleton with 104,886 voxels. To assess the longitudinal effects of therapy, three interactions with time from the model were examined. First, β3, the general effect of time was evaluated, where 8,248 voxels had a significant change over time. A positive beta, meaning increasing FA, was observed in 8,149 of the significant voxels (Figure 2). Next, β5, the interaction between time and the radiation dose received at each pixel was examined. Here, 2,788 voxels were significant, and 2,759 of these voxels had a negative beta, or a decreasing FA (Figure 3). Finally, the interaction between baseline age and time was examined, but there were no significant voxels in this analysis. Comparing the location of these significant voxels to the JHU atlas (Table 1), the effect of increasing FA over time with a decreased FA due to dose was seen in the corpus callosum, cerebral peduncle, internal capsule, corona radiata, posterior thalamic radiation, external capsule, and the superior longitudinal fasciculus.DISCUSSION
This study continues to expand our knowledge of the effects of current pediatric medulloblastoma therapy on the immature brain. Previous work has shown a level of damage due to surgery alone, and additional damage is expected immediately after radiation therapy. The results presented in this study suggest that there is an overall recovery of FA values over the next 18 months, and this recovery is hampered in areas of increased radiation dose. These results are consistent with white matter volume changes observed in pre-clinical models.10 The results identified several white matter tracts that are particularly vulnerable to damage. It will be important to associate recovery of FA with neurocognitive performance in these subjects.CONCLUSION
FA values recover after the administration of radiation therapy with further treatment and follow-up, but this recovery is attenuated by higher radiation doses. Tumor and therapy-related longitudinal changes in FA need to be considered when conducting cross-sectional studies of patients at time points during or after therapy for medulloblastoma.Acknowledgements
We acknowledge the valuable contributions of Rhonda Simmons and Kathy Jordan, advanced signal processing technicians, and funding in part by the Cancer Center Support Grant P30 CA-21765 from the National Cancer Institute, and ALSAC.
References
1. Gajjar A, Robinson GW, Smith KS, et al. Outcomes by Clinical and Molecular Features in Children With Medulloblastoma Treated With Risk-Adapted Therapy: Results of an International Phase III Trial (SJMB03). J Clin Oncol. 2021; 39(7):822-835.
2. Glass JO, Ogg RJ, Hyun JW, et al. Disrupted development and integrity of frontal white matter in patients treated for pediatric medulloblastoma. Neuro Oncol. 2017; 19(10):1408-1418.
3. Acharya S, Guo Y, Patni T, et al. Association Between Brain Substructure Dose and Cognitive Outcomes in Children With Medulloblastoma Treated on SJMB03: A Step Toward Substructure-Informed Planning. J Clin Oncol. 2021:JCO2101480.
4. Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004; 23 Suppl 1:S208-219.
5. Andersson JL, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage. 2003; 20(2):870-888.
6. Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006; 31(4):1487-1505.
7. Gaylor DW, Hopper FN. Estimating the Degrees of Freedom for Linear Combinations of Mean Squares by Satterthwaite's Formula. Technometrics. 1969; 11(4):691-706.
8. Benjamini Y, Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. J R Stat Soc B. 1995; 57(1):289-300.
9. Yu HJ, Christodoulou C, Bhise V, et al. Multiple white matter tract abnormalities underlie cognitive impairment in RRMS. Neuroimage. 2012; 59(4):3713-3722.
10. Nieman BJ, de Guzman AE, Gazdzinski LM, et al. White and Gray Matter Abnormalities After Cranial Radiation in Children and Mice. Int J Radiat Oncol Biol Phys. 2015; 93(4):882-891.
Figures
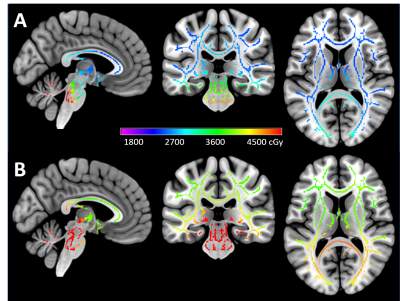
Figure 1. Example dosimetry for an average-risk (A) and high-risk (B) patient mapped in cGy on the TBSS skeleton.
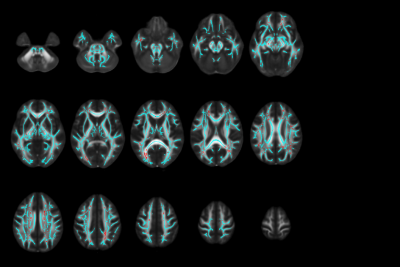
Figure 2. Significant voxels with positive change over time after radiation therapy. The FMRIB58_FA standard space in MNI coordinates is overlaid by the skeleton in cyan, and red indicates the location of voxels with positive change over time that were significant. This finding demonstrates a recovery of the FA after irradiation.
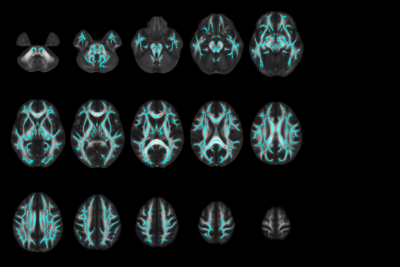
Figure 3. Significant voxels associated with dose and negative change over time after radiation therapy. The FMRIB58_FA standard space in MNI coordinates is overlaid by the skeleton in cyan, and red indicates the location of voxels with negative change over time associated with dose that were significant. These findings demonstrate an attenuation of FA recovery after irradiation with higher doses of radiation.
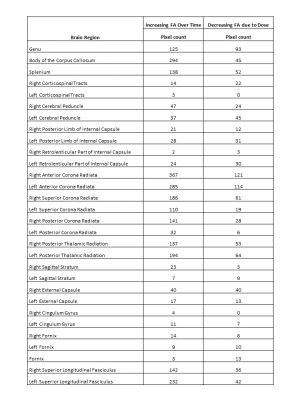
Table 1 The location of the significant voxels based on the JHU-ICBM-DTI-81 WM Atlas. Not all regions of the skeleton were covered by the atlas.