1240
Prenatal and Postnatal Cerebellar Development
Elizabeth Weisse1, Yaqing Chen2, Alvaro Gajardo Cataldo2, Hans-Georg Müller2, Changbo Zhu2, Jonathan O’Muircheartaigh3, Maximilian Pietsch4, Sian Wilson4, Kristofer Bouchard5,6, Sylvia Madhow5,6, James Cole7,8, Francesca Biondo7,9, Viren D’Sa10,11, Sean C. L. Deoni11,12,13, and Muriel M.K. Bruchhage1,11,14
1Department of Diagnostic Imaging, Rhode Island Hospital, Providence, RI, United States, 2Department of Statistics, UC Davis, Davis, CA, United States, 3Department of Forensic & Neurodevelopmental Sciences, King's College London, London, United Kingdom, 4Centre for the Developing Brain, Department of Perinatal Imaging and Health, King's College London, London, United Kingdom, 5Scientific Data Division & Biological Systems and Engineering Division, LBNL, Berkeley, CA, United States, 6Helen Wills Neuroscience Institute & Redwood Center for Theoretical Neuroscience, UC Berkeley, Berkeley, CA, United States, 7Centre for Medical Image Computing, Computer Science, UCL, London, United Kingdom, 8Dementia Research Centre, Queen Square Institute of Neurology, UCL, London, United Kingdom, 9Department of Neuroimaging, Institute of Psychiatry, Psychology and Neuroscience, UCL, London, United Kingdom, 10Department of Pediatrics, Rhode Island Hospital, Providence, RI, United States, 11Department of Pediatrics, Warren Alpert Medical School at Brown University, Providence, RI, United States, 12Brown's Advanced Baby Imaging Lab, Rhode Island Hospital, Providence, RI, United States, 13MRI Research, Department of Diagnostic Imaging, Rhode Island Hospital, Providence, RI, United States, 14Institute of Social Sciences, Department of Psychology, University of Stavanger, Stavanger, Norway
1Department of Diagnostic Imaging, Rhode Island Hospital, Providence, RI, United States, 2Department of Statistics, UC Davis, Davis, CA, United States, 3Department of Forensic & Neurodevelopmental Sciences, King's College London, London, United Kingdom, 4Centre for the Developing Brain, Department of Perinatal Imaging and Health, King's College London, London, United Kingdom, 5Scientific Data Division & Biological Systems and Engineering Division, LBNL, Berkeley, CA, United States, 6Helen Wills Neuroscience Institute & Redwood Center for Theoretical Neuroscience, UC Berkeley, Berkeley, CA, United States, 7Centre for Medical Image Computing, Computer Science, UCL, London, United Kingdom, 8Dementia Research Centre, Queen Square Institute of Neurology, UCL, London, United Kingdom, 9Department of Neuroimaging, Institute of Psychiatry, Psychology and Neuroscience, UCL, London, United Kingdom, 10Department of Pediatrics, Rhode Island Hospital, Providence, RI, United States, 11Department of Pediatrics, Warren Alpert Medical School at Brown University, Providence, RI, United States, 12Brown's Advanced Baby Imaging Lab, Rhode Island Hospital, Providence, RI, United States, 13MRI Research, Department of Diagnostic Imaging, Rhode Island Hospital, Providence, RI, United States, 14Institute of Social Sciences, Department of Psychology, University of Stavanger, Stavanger, Norway
Synopsis
The cerebellum is one of the earliest structures to develop prenatally and is documented to play an important role in motor development and, more recently, cognitive development. We investigated cerebellar development and its contribution to later overall (ELC), nonverbal (NVDQ) and verbal (VDQ) cognitive development from 22 weeks of gestation to 15 years of age. Total postnatal cerebellar volume significantly increased with ELC and VDQ but decreased with NVDQ, while prenatal cerebellar volume significantly increased with ELC with age. This could be indicative of the cerebellum’s contribution to overall cognitive development refining with development and age.
Introduction
The human cerebellum is one of the earliest brain regions to develop prenatally, but continues to grow until late adolescence and adulthood1. While the cerebellum’s role in motor control is well recognized, evidence for its contribution to the development of cognitive function is being increasingly documented both structurally2 and functionally3. The prenatal phase plays a crucial role in postnatal brain and skill development. During prenatal development, neurulation results in the formation of the central nervous system, the brain and spinal cord. Here, prenatal brain development follows distinct phases and timing, with cerebellar development preceding neocortical development1. At week 7 after gestation during prenatal development, the superior cerebellar peduncle begins to develop linking the cerebellum with the neocortex, and in turn allow both structures to continue their development in tandem. Throughout pre- and postnatal development, cerebellar volume and white matter tracts continue to grow and refine into adolescence and adulthood4.Insults and deviations during prenatal, perinatal and early postnatal cerebellar development make this developmental phase especially vulnerable for malformation, disorder and disease5, including neurodevelopmental disorders such as autism spectrum disorder6. Insults to the prenatal cerebellum such as through prenatal alcohol exposure can result in significant deformity in the cerebellum, in turn impacting cognitive development and verbal learning7. However, while recent research has demonstrated the cerebellum’s importance to postnatal motor and cognitive development, no study has investigated and compared its pre- and postnatal contribution to overall, verbal and nonverbal cognitive development.
Methods
Longitudinal data for fetal head circumference (HC) and cerebellar size8 were acquired for the prenatal cohort (PreC) consisting of 431 children (46% male) at weeks 22, 28, and 34 of gestation on a GE Voluson E8 ultrasound system. Postnatal cohort (PostC) sMRI data was acquired for 606 term-born typically developing children (56% male, 2-180 months of age, 42 mo StDev) at a 3T Siemens Trio scanner with the following parameters: TE=5.6, TR=1400, FA=15, 1.1x1.1x1.1mm resolution, 160x160 matrix, 112 slices.Using a combination of linear and nonlinear image registration, we created representative age-specific templates using the ANTs package9 for the Resonance and BCP datasets separately. After age specific templates were created, a single flow from each age to the 12-month template was estimated and a final warp from the 12-month template to standard adult MNI space was performed and the Harvard-Oxford brain atlas applied10. In order to increase comparability of potential results, we combined cerebellar subregions to one total cerebellar volume. PACE brain-for-age growth percentiles were created for HC, as well as cerebellar volume pre- and postnatally11. We used z-score normed Mullen Scales of Early Learning12 to assess overall cognitive development (early learning composite, ELC), nonverbal development quotient (NVDQ) and verbal development quotient (VDQ) at the assessment closest to birth (PreC) or at the time of scan (PostC).
Univariate ANOVAs and growth regression plots were performed using SPSS (IBM SPSS Statistics vs 28.0) for all postnatal data and weighted for the 3 age groups where most children were assessed prenatally (22, 28 and 34 weeks of gestation). Statistical significance was set at p≤.05 and all analyses were corrected for age in days of gestation (PreC) or corrected for full-term gestation (PostC).
Results
PACE growth percentiles were constructed for the cerebellum and HC volume (Figure1). Prenatal ANOVAs showed statistically significant intercepts of increasing cerebellar size and ELC (F=5.09, p=.026), and nearly reached statistically significance for VDQ (F=3.91, p=0.51) and NVDQ (F=3.89, p=0.52) intercepts. Postnatally, ANOVAs reached significance for increasing cerebellar volume with ELC (F=6.618, p=0.10), VDQ (F=6.634, p=0.10) and NVDQ (F=4.10, p=0.43). Growth regression plots were created to demonstrate direction (Figure2) but only reached statistical significance for PostC (rho(NVDQ)=0.16; rho(VDQ)=.40; rho(ELC)=.21: all p≤.05).Discussion
In this large pediatric study, we have created pre- and postnatal growth percentiles for cerebellar volumes (Figure1) and linked pre- and postnatal cerebellar development with overall, nonverbal and verbal cognitive development. Interestingly, while prenatally overall cognitive development was significantly associated with increasing cerebellar volume over time, postnatally only ELC and VDQ but not NVDQ increased with increasing cerebellar volume (Figure2). Indeed, in one of our previous studies, we have shown plastic white and grey matter changes after learning how to drum, supporting increasing evidence that the cerebellum decreases in volume and functional connectivity after a new skill is learned13, opposite to the neocortex. This is supported by preclinical studies demonstrating Purkinje cells recognizing certain contexts when an action is being performed and then creating an anticipatory neural state14-16. Thus, our findings could indicate a shift towards supporting more demanding skill acquisition with age, shifting from (learned) nonverbal skills during early childhood to newer and demanding verbal skill development, as reflected in the decrease of cerebellar volume with VDQ but not NVDQ with age (Figure2).While promising, we acknowledge the limitations of our study associated with methods and design used. Sonographic assessment during the first trimester is recognized as the most accurate estimate of gestational age and fetal size8 and ultrasound measures of the cerebellum have shown comparable precision measures for ultrasound measurements when compared with microMRI17. However, future studies should replicate our findings using longitudinal pre- to postnatal MRI datasets, optimally supported by continuous 3D ultrasound measures.
Acknowledgements
We would like to thank the Bill and Melinda Gates Foundation (INV005774) for their continuous support of this work.References
- Silbereis, J. C., Pochareddy, S., Zhu, Y., Li, M., & Sestan, N. (2016). The cellular and molecular landscapes of the developing Human Central Nervous System. Neuron, 89(2), 248–268.
- Schmahmann, J. D. (2019). The cerebellum and cognition. Neuroscience Letters, 688, 62-75.
- Bruchhage et al., (2020). Functional connectivity correlates of infant and early childhood cognitive development. Brain Structure and Function, 255(2), 669-681.
- Bostan, A. C., Dum, R. P., Strick, P. L. (2013). Cerebellar networks with the cerebral cortex and basal ganglia. Trends Cogn Sci., 17, 241-254.
- Tavano, A., Grasso, R., Gagliardi, C., Triulzi, F., Bresolin, N., Fabbro, F., & Borgatti, R. (2007). Disorders of cognitive and affective development in cerebellar malformations. Brain, 130(10), 2646–2660.
- Bruchhage et al., (2018). Cerebellar involvement in autism and ADHD. The Cerebellum: Disorders and Treatment, 61-72.
- Nunez, C. C., Roussotte, F., & Sowell, E. R. (2011). Focus on: structural and functional brain abnormalities in fetal alcohol spectrum disorders. Alcohol research & health : the journal of the National Institute on Alcohol Abuse and Alcoholism, 34(1), 121–131.
- Shi, Y., Xue, Y., Chen, C., Lin, K., & Zhou, Z. (2020). Association of gestational age with MRI-based biometrics of brain development in fetuses. BMC Medical Imaging, 20(1).
- Avants et al. (2014). The insight ToolKit image registration framework. Front. Neuroinformatics. 28,8:44.
- Jenkinson, M., Beckmann, C.F., Behrens T.E.J., Woolrich, M.W., Smith, S.M. (2012). FSL. NeuroImage, 62(2), 782-790.
- Chen, Y, Dubey, P, Müller, H-G, Bruchhage, MMK, Wang, J-l, Deoni, SCL (2021). Modeling sparse longitudinal data in early neurodevelopment. NeuroImage, 237:118079.
- Mullen, EM (1995). Mullen scales of early learning. AGS; Circle Pines, MN.
- Bruchhage, M. M., Amad, A., Draper, S. B., Seidman, J., Lacerda, L., Laguna, P. L., Lowry, R. G., Wheeler, J., Robertson, A., Dell’Acqua, F., Smith, M. S., & Williams, S. C. (2020). Drum training induces long-term plasticity in the cerebellum and connected cortical thickness. Scientific Reports, 10(1).
- Marr, D. (1969). A theory of cerebellar cortex. The Journal of Physiology, 202(2), 437–470.
- Albus, J. S. (1971). A theory of cerebellar function. Mathematical Biosciences, 10(1-2), 25–61.
- Ito, M. (1972). Neural design of the Cerebellar Motor Control System. Brain Research, 40(1), 81–84.
- Vulturar, D., Fărcăşanu, A., Turcu, F., Boitor, D., & Crivii, C. (2018). The volume of the cerebellum in the second semester of gestation. Medicine and Pharmacy Reports, 91(2), 176–180.
Figures
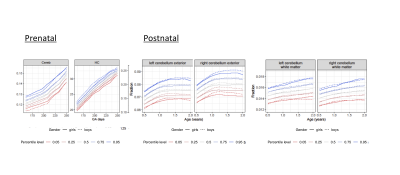
Figure 1. Age-based dynamic percentiles of brain regions corrected for total intracranial volume estimated by local Fréchet regression for pre-and postnatal data.
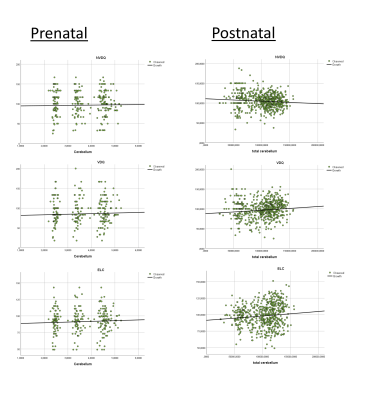
Figure 2. Regression growth plots for z-scores of overall cognitive development (early learning composite, ELC), nonverbal (verbal development quotient, VDQ) and nonverbal development (nonverbal developmental quotient, NVDQ) for both cohorts.
DOI: https://doi.org/10.58530/2022/1240