1238
MRI assessment of cerebral oxygen delivery before and after surgery in infants with congenital heart disease1Centre for the Developing Brain, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2Neonatal Intensive Care Unit, Evelina London Children's Hospital, London, United Kingdom, 3Department of Cardiovascular Imaging, School of Biomedical Engineering & Imaging Science, King's College London, London, United Kingdom, 4Department of Congenital Heart Disease, Evelina London Children's Hospital, London, United Kingdom
Synopsis
Reduced cerebral oxygen delivery (CDO2) in infants with congenital heart disease (CHD) is associated with abnormal brain development. We investigated how cerebral blood flow, haemoglobin, and oxygen saturations, which all contribute to CDO2, change following surgery in a cohort of 24 infants with CHD. There was no significant change in CDO2 after surgery (P = 0.62). However, both cerebral blood flow (P < 0.001) and pre-ductal oxygen saturations (P = 0.008) improved significantly, whilst haemoglobin dropped significantly (P < 0.001). Consistent with previous reports, the changes that occur in cerebral blood flow following surgery are dependent on the type of cardiac lesion.
Introduction
Congenital heart disease (CHD) is the commonest group of congenital anomalies. Most infants born with CHD now survive. However, survivors of CHD are at increased risk of neurodevelopmental impairment. The mechanisms underlying these impairments are unclear and are likely to be multifactorial. Previous research has highlighted that reduced cerebral oxygen delivery (CDO2) prior to surgery in infants with CHD is associated with impaired brain development, including reduced grey matter volume, cortical gyrification and altered cortical microstructure (1,2). There is currently limited understanding of how CDO2 changes following cardiac surgery. We aimed to investigate CDO2 in infants with CHD before and after corrective surgical procedures.Methods
Infants with CHD were recruited after birth as part of the Congenital Heart Disease Imaging Programme. MRI scans were performed on a Philips 3T MRI scanner and included T1-weighted, T2-weighted and diffusion-weighted imaging, and phase-contrast angiography. For this, quantitative flow imaging was performed using velocity sensitised phase contrast imaging, with a single-slice T1-weighted fast field echo sequence (TR: 6.4 ms, TE: 4.3 ms, Resolution 0.6x0.6x4mm2, flip-angle 10°, 20 repetitions, maximal encoding velocity 140 cm s-1).Participants were included in this study if they underwent cardiac surgery requiring cardiopulmonary bypass or a corrective procedure (i.e., valve dilation), had both pre- and post-operative MRI scans with good quality PCA, and haemoglobin levels and pre-ductal oxygen saturations were recorded at both scans.
CDO2 was calculated using: CDO2 = SaO2 x [Hb] (g dL-1) x 1.36 × [CBF] (ml min-1)
Where SaO2 is the pre-ductal saturation at the scan, Hb is the haemoglobin close to the time of the scan, 1.36 is the amount of oxygen bound per gram of haemoglobin at 1 atmosphere (Hüfner’s constant) (3) and CBF is Cerebral Blood Flow. CBF was calculated from the PCA images, using the Segment software package (4) to determine blood flow in the left internal carotid, right internal carotid and basilar arteries.
Paired T-tests were used to compare parametric data. Wilcoxon signed rank tests were used to compare non-parametric data.
Results
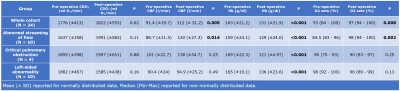
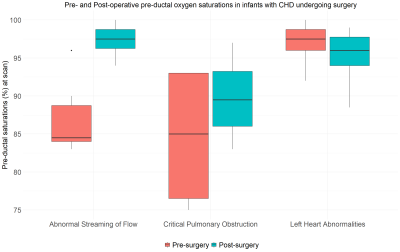
A total of 24 infants (13 male) were included, comprising three diagnostic groups: abnormal streaming of flow (N=10, all with transposition of the great arteries); critical pulmonary obstruction (N = 4: 3 with pulmonary atresia, 1 with tetralogy of Fallot); and left-sided heart abnormalities (N = 10: 9 with coarctation of the aorta, 1 with hypoplastic left heart syndrome). Median age at birth was 38+4 weeks (range: 35+2 to 40+0), median age at pre-surgical scan was 39+3 weeks (range: 36+3 to 41+1), median age at surgery was 40+2 weeks (range: 37+0 to 46+7) and median age at post-surgical scan was 41+6 weeks (range: 37+7 to 47+7).Pre- and post-operative values for CDO2, CBF, haemoglobin and pre-ductal oxygen saturations for the entire cohort, and within each of the three diagnostic groups are shown in Figure 2.
There was no significant change in CDO2 pre- and post-surgery (P = 0.62). Both CBF (P < 0.001) and pre-ductal oxygen saturations (P = 0.008) improved significantly after surgery, whilst haemoglobin dropped significantly (P < 0.001).
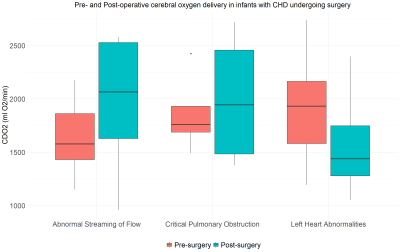
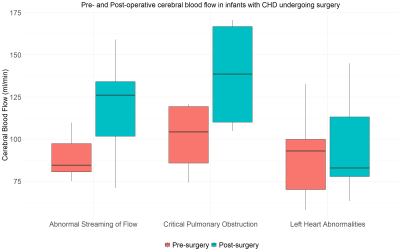
There was a significant increase in CBF after surgery in the group with abnormal streaming of flow (P = 0.005), but not the other two groups. Hb decreased significantly after surgery in all three groups. Pre-ductal oxygen saturations increased significantly in the group with abnormal streaming of flow (P = 0.002) but not in the other two groups. Figures 3-5 highlight how CDO2, CBF and Hb compare pre- and post-surgery in all three diagnostic groups.
Discussion
Previous studies have highlighted that lower CDO2 before surgery is associated with abnormal brain development in infants with CHD. The aim of this study was to improve our understanding of changes in CDO2 before and after corrective surgery. As CDO2 encompasses measures of oxygen saturation, haemoglobin and CBF, changes in CDO2 may reflect changes in any of these.We observed a decrease in haemoglobin after surgery, consistent with the maturational decrease in HB observed in infancy (5), and likely reflecting some of the expected blood loss during surgery.
Consistent with previous reports (6), the changes that occur in CBF following surgery are dependent on the type of cardiac lesion.
In infants with abnormal streaming of flow, following correction of the underlying anatomical abnormality, the increase in CDO2 was associated with a significant improvement in both their pre-ductal oxygen saturations and CBF. The decrease in CDO2 in the group with ‘left-sided heart abnormalities’, (composed almost entirely of infants with coarctation of the aorta), may be because their pre-operative oxygen saturations are relatively normal and so can't be improved, and haemoglobin falls in this period. In infants with critical pulmonary obstruction, the only change observed after surgery was a reduction in haemoglobin, although the small sample size (N=4) in this group makes it difficult to draw reliable conclusions here.
Conclusion
Changes in cerebral oxygen delivery in infants with CHD undergoing corrective surgery are related to their underlying cardiac physiology, the effect of corrective surgery on cerebral blood flow and, in the acute post-surgical period, an expected drop in haemoglobin. Further studies are required to investigate whether any change in CDO2 following surgery is associated with later neurodevelopmental outcomes.Acknowledgements
This work was supported by the Medical Research Council UK (MR/L011530/1 and MR/V002465/1), the British Heart Foundation (FS/15/55/31649), and Action Medical Research (GN2630). This research was supported by core funding from the Wellcome/EPSRC Centre for Medical Engineering (WT 203148/Z/16/Z), MRC strategic grant (MR/K006355/1), Medical Research Council Centre grant (MR/N026063/1), and by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and Kings College London.References
1 - Kelly CJ et al. Impaired development of the cerebral cortex in infants with congenital heart disease is correlated to reduced cerebral oxygen delivery. Sci Rep. 2017 Dec;7(1):15088.
2 - Kelly CJ et al. Abnormal microstructural development of the cerebral cortex in neonates with congenital heart disease is associated with impaired cerebral oxygen delivery. Journal of the American Heart Association. 2019 Mar 5;8(5):e009893.
3 - Lim, J. M. et al. Cerebral oxygen delivery is reduced in newborns with congenital heart disease. J. Torac. Cardiovasc. Surg. 152, 1095–103. 2016.
4 - Heiberg, E. et al. Design and validation of Segment - freely available sofware for cardiovascular image analysis. BMC Med. Imaging 10, 1 (2010).
5 - Jopling J et al. Reference ranges for hematocrit and blood hemoglobin concentration during the neonatal period: data from a multihospital health care system. Pediatrics. 2009 Feb;123(2):e333-7. doi: 10.1542/peds.2008-2654.
6 - Cheng H et al. 2020. Abnormalities in cerebral hemodynamics and changes with surgical intervention in neonates with congenital heart disease. The Journal of thoracic and cardiovascular surgery, 2020. 159(5), pp.2012-2021.
Figures