1237
Reduced neonatal fronto-limbic connectivity is associated with higher externalizing symptoms in toddlers with Congenital Heart Disease1Centre for the Developing Brain, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2Trinity College Institute of Neuroscience and Cognitive Systems Group, Discipline of Psychiatry, School of Medicine, Trinity College Dublin, Dublin, Ireland, 3Department of Electrical Engineering (ESAT/PSI), KU Leuven, Leuven, Belgium, 4Department for Forensic and Neurodevelopmental Sciences, Institute of Psychiatry, Psychology and Neuroscience, King's College London, London, United Kingdom, 5Biomedical Image Technologies, ETSI Telecomunicación, Universidad Politécnica de Madrid & CIBER-BBN, Madrid, Spain, 6Biomedical Engineering Department, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 7Paediatric Cardiology Department, Evelina London Children's Healthcare, London, United Kingdom, 8Department of Child and Adolescent Psychiatry, Institute of Psychiatry, Psychology and Neuroscience, King's College London, London, United Kingdom
Synopsis
Infants with Congenital Heart Disease (CHD) are at increased risk of neurodevelopmental impairments. Forty-three neonates with CHD underwent multi-shell high angular resolution diffusion MRI (HARDI) on a 3T scanner before surgery. At 22 months parents completed a questionnaire characterising internalizing and externalizing behaviours. Network-based statistics were used to characterise the relationship between internalizing and externalizing symptoms and structural connectivity. Reduced neonatal structural connectivity in fronto-limbic regions before surgery was associated with increased externalizing symptomatology in toddlers with CHD.
Introduction
Infants with Congenital Heart Disease (CHD) are at risk of impaired neurodevelopment, the origins of which are not well understood1. The aim of this study was to characterise the relationship between neonatal structural connectivity soon after birth and behavioural outcomes at 22 months.Methods
Forty-three infants [21 male; median (range) gestational age at birth = 38.57 (34.86-41.57) weeks; postmenstrual age at scan median (range) = 39.29 (36.43-41.86) weeks] with serious or critical CHD (summarised in Table 1) underwent brain MRI before surgery on a Phillips Achieva 3T scanner situated on the neonatal unit at St Thomas’ Hospital, London. High angular resolution diffusion MRI (HARDI)2 was acquired for all infants (TR/TE 3.8s/90ms, b = 0s/mm2 (n = 20), b = 400s/mm2 (n = 64), b = 1000s/mm2 (n = 88), b = 2600s/mm2 (n = 128), multiband factor = 4, resolution: 1.5 × 1.5 × 3mm with 1.5mm slice overlap with interleaved phase encoding, reconstructed voxel size 1.5mm isotropic). HARDI data underwent denoising3, Gibbs ringing suppression4, and correction for motion and image distortion using spherical harmonics and radial decomposition (SHARD)5.To assess network organisation, we constructed structural connectivity networks for each infant using a neonatal adaptation6 of the automated anatomical labelling atlas7 as nodes and SIFT2 weights8 of streamlines generated in MRtrix39 connecting each region as edges. We calculated global network features (global network density, average strength, global efficiency, and local efficiency) using the Brain Connectivity Toolbox (BCT)10. Edge-wise associations between structural connectivity and behavioural scores was assessed using the network-based statistics (NBS) toolbox11. The t-statistic threshold was set at 3.1 where connections exceeding this value were considered significant. NBS results are dependent on t-statistic threshold, so analyses were rerun at t = 2.5–3.5.
Infants underwent a neurodevelopmental assessment at a median 22.1 months (range 20.3-37.2) corrected age. Cognitive abilities were assessed with the Bayley-Scales of Infant and Toddler Development 3rd Edition. Parents completed the Child Behaviour Checklist 1.5-5 years questionnaire and internalizing and externalizing scores were calculated (higher scores indicate increased symptomatology). The index of multiple deprivation (IMD) was calculated from postcode at birth for each infant as a measure of socioeconomic status. It was not possible to calculate IMD for one infant with CHD.
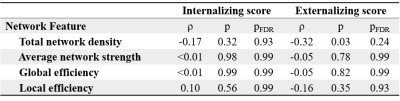
Partial Spearman’s rank correlations were used to assess the association between global network features and internalizing and externalizing scores covarying for postmenstrual age at scan, gestational age at birth, sex, brain injury severity, cognitive composite score and IMD. P-values underwent false discovery rate correction and were reported as pFDR.
For the NBS analysis, general linear models were generated to test for positive and negative relationships between internalizing and externalizing scores and structural connectivity controlling for sex, gestational age at birth, postmenstrual age at scan, brain injury severity, cognitive composite score and index of multiple deprivation. Models were run with 10,000 permutations and controlled the family-wise error rate (significance threshold set at p< 0.025).
Results
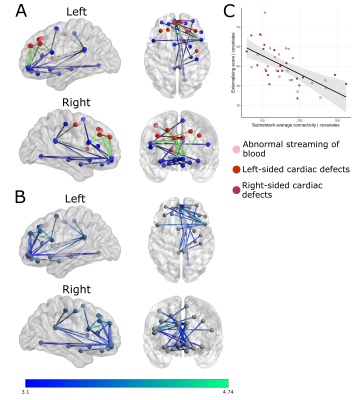
NBS revealed a fronto-limbic network of 26 nodes sharing 29 edges (Table 2) where reduced connectivity was associated with higher externalizing scores in infants with CHD (Figure 1). No networks with significantly increased connectivity were associated with higher externalizing scores. No networks were associated with internalizing scores. There were also no significant associations between global network features and internalizing or externalizing scores (Table 3).Table 2 lists edges negatively associated with externalizing scores and the associated t-statistic at a threshold of t=3.1. Sensitivity analysis revealed no significant networks at t=2.5-2.8. Significant edges were identified in the fronto-limbic network at t=2.9-3.5.
Discussion
Young children with CHD are at increased risk of both internalizing and externalizing behaviour problems12. This study provides evidence that altered neonatal structural connectivity in a fronto-limbic subnetwork before surgery is associated with increased externalizing symptoms in toddlers with CHD.Corticolimbic networks are implicated in internalizing and externalizing symptoms across childhood13. Lower clustering coefficient in the right amygdala during the neonatal period has been associated with increased internalizing and externalizing symptoms at two years in healthy infants14. In healthy children and adolescents, externalizing symptoms have been linked to structure and function within amygdala-orbitofrontal circuitry15,16,17. Taken together, these data point to the importance of fronto-limbic circuitry in the development of externalizing behaviours across childhood.
Conclusions
Reduced structural connectivity in a fronto-limbic network at birth may underlie externalizing symptoms in children with CHD.Acknowledgements
This work was supported by the Medical Research Council UK (MR/L011530/1 and MR/V002465/1), the British Heart Foundation (FS/15/55/31649), and Action Medical Research (GN2630). This research was supported by core funding from the Wellcome/EPSRC Centre for Medical Engineering (WT 203148/Z/16/Z), MRC strategic grant (MR/K006355/1), Medical Research Council Centre grant (MR/N026063/1), and by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and Kings College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and social care. DC is supported by the Flemish Research Foundation (FWO; grant number 12ZV420N). MP is funded in part by the Bill & Melinda Gates Foundation (INV-005774).References
1. Marino BS, Lipkin PH, Newburger JW et al. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the American Heart Association. Circulation 2012; 126: 1143–72.
2. Hutter J, Tournier J-D, Price AN, et al. Time-efficient and flexible design of optimized multishell HARDI diffusion: Time-Efficient Flexible dMRI. Magn. Reson. Med. 2018;79(3):1276–1292.
3. Cordero-Grande L, Christiaens D, Hutter J, et al. Complex diffusion-weighted image estimation via matrix recovery under general noise models. NeuroImage 2019; 200: 391–404.
4. Kellner E, Dhital B, Kiselev VG, et al. Gibbs-ringing artifact removal based on local subvoxel-shifts: Gibbs-Ringing Artifact Removal. Magnetic Resonance in Medicine. 2016; 76(5): 1574–1581.
5. Christiaens D, Cordero-Grande L, Pietsch M et al. Scattered slice SHARD reconstruction for motion correction in multi-shell diffusion MRI. Neuroimage. 2021; 225:117437.
6. Shi F, Yap P-T, Wu G, et al. Infant Brain Atlases from Neonates to 1- and 2-Year-Olds. PLoS One. 2011;6.
7. Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated Anatomical Labeling of Activations in SPM Using a Macroscopic Anatomical Parcellation of the MNI MRI Single-Subject Brain. NeuroImage. 2002;15(1):273–289.
8. Smith RE, Tournier J-D, Calamante F, et al. SIFT2: Enabling dense quantitative assessment of brain white matter connectivity using streamlines tractography. NeuroImage. 2015; 119: 338–351.
9. Tournier J-D, Smith R, Raffelt D, et al. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. NeuroImage. 2019; 202: 116137.
10. Rubinov M, Sporns O. Complex network measures of brain connectivity: Uses and interpretations. NeuroImage. 2010;52(3):1059–1069.
11. Zalesky A, Fornito A, Bullmore ET. Network-based statistic: Identifying differences in brain networks. NeuroImage. 2010;53(4):1197–1207.
12. Clancy T, Jordan B, de Weerth C, et al. Early Emotional, Behavioural and Social Development of Infants and Young Children with Congenital Heart Disease: A Systematic Review. Journal of Clinical Psychology in Medical Settings. 2020; 27: 686-703.
13. Tucker DM, Poulsen C, Luu P. Critical periods for the neurodevelopmental processes of externalizing and internalizing. Development and Psychopathology. 2015; 27(2):321-46.
14. Wee C-Y, Tuan TA, Broekman BFP et al. Neonatal neural networks predict children behavioral profiles later in life. Hum Brain Mapp. 2017; 38(3): 1362-1373.
15. Ameis SH, Ducharme S, Albaugh MD, et al. Cortical Thickness, Cortico-Amygdalar Networks, and Externalizing Behaviors in Healthy Children. Biological Psychiatry. 2014; 75(1): 65-72.
16. Thijssen S, Collins PF, Weiss H, et al. The longitudinal association between externalizing behavior and frontoamygdalar resting‐state functional connectivity in late adolescence and young adulthood. J Child Psychol Pyschiatry. 2021; 62(7): 857-867.
17. Andre QR, Geeraert BL, Lebel C. Brain structure and internalizing and externalizing behavior in typically developing children and adolescents. Brain Structure and Function. 2020; 225: 1369-1378.
18. Ni Bhroin M, Abo Seada S, Bonthrone AF, et al. Reduced structural connectivity in cortico-striatal-thalamic network in neonates with congenital heart disease. Neuroimage Clinical. 2020; 28: 102423.
Figures