1233
Dynamic resting state connectivity of the default mode, salience and central executive networks in adolescents with concussion1Psychology, Neuroscience & Behaviour, McMaster University, Hamilton, ON, Canada, 2Department of Psychiatry, Western University, London, ON, Canada, 3School of Rehabilitation Science, McMaster University, Hamilton, ON, Canada, 4Department of Electrical & Computer Engineering, McMaster University, Hamilton, ON, Canada
Synopsis
Our study evaluated the dynamic functional connectivity of adolescents with concussion in comparison to healthy controls using (1) sliding window ROI-to-ROI and (2) sliding window graph theory analyses. Adolescents with concussion exhibited higher between-network connectivity between the salience network and central executive network, but reduced between-network connectivity between the default mode network to both the salience and executive networks. This suggests lower default mode network integration and engagement during rest following concussion in adolescents.
Background
Menon’s triple network framework of neuropathology suggests that the default mode (DMN), salience (SN), and central executive (CEN) networks shed light on cognitive and emotional dysfunction of several neurological disorders 1,2. Concussion, like other neuropathological disorders and conditions, may also lead to aberrant between-network connectivity in the DMN, SN and CEN 3–5. Evidence suggests that concussion during childhood decreases the brain’s ability to flexibly transition between network states 6; however, minimal research has specifically evaluated the dynamic connectivity of the DMN, SN and CEN in adolescents.Methods
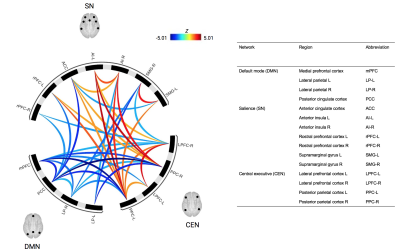
Thirty-four adolescents with a concussion diagnosis were scanned within two months of injury (age 10-18 years; 12 males and 22 females; median number of days since injury = 30.5). All adolescents with concussion were still symptomatic at the time of scanning. A 6-minute resting state scan was completed on a 3T GE MRI (TE/TR=35/2000 ms, temporal points = 180, flip angle = 90o, image matrix = 64 x 64, slice thickness = 3 mm, FOV = 220 mm, 35 slices). Thirty-four age-matched controls were obtained from the publicly available Autism Brain Imaging Data Exchange (ABIDE) 7 database from which healthy controls had no history of head trauma (3T GE MRI, TE/TR=30/2000 ms, temporal points = 300, flip angle = 90o, image matrix = 64 x 64, slice thickness = 3 mm, FOV = 220 mm, 40 slices).Resting state analysis was conducted using CONN Toolbox 20b 8 using a sliding window approach (18 windows; 30s blocks; 20s onsets). Functional connectivity metrics were extracted from ROI-to-ROI and graph theory analyses. ROIs from the DMN, SN and CEN were provided by the CONN Toolbox (105 connections; 15 ROIs). Corrections for multiple comparisons were done using parametric multivariate statistics 9 with a cluster threshold of p-FDR < 0.05 (MVPA omnibus test) and a connection threshold of p-uncorrected < 0.05.
Results and Discussion
The sliding window ROI-to-ROI analysis revealed that the temporal average of functional connectivity was statistically different between groups. Participants with concussion had significantly greater connectivity between CEN-SN regions compared to controls, but significantly less connectivity within the DMN, between DMN-CEN, and between DMN-SN, relative to controls (Figure 1). Reduced DMN-SN cross-communication might illustrate why there are often reported increases in cognitive and emotional issues following injury. Heightened SN-CEN connectivity might suggest a tendency to recruit the CEN regions. This could imply a hypervigilant state, even during rest, post-concussion.The sliding window graph theory analysis revealed that the temporal average was statistically different between groups such that participants with concussion had significantly decreased global efficiency (t(66) = -3.76, p-FDR = 0.005), cost (t(66) = -4.53, p-FDR = 0.0004), and degree (t(66) = -4.53, p-FDR = 0.0004) compared to controls in the posterior cingulate cortex (PCC) of the DMN. The sliding window graph theory analysis also revealed that the temporal variability was different between groups such that participants with concussion had significantly decreased cost and degree (t(66) = -3.08, p-FDR = 0.045878) compared to controls in the medial prefrontal cortex (mPFC) of the DMN. This might indicate a deficit in the mPFC to flexibly connect and disconnect with other nodes of the network (i.e., a more rigid network connectivity pattern).
Conclusion
Following concussion, adolescents display aberrant dynamic connectivity between the default mode, salience and central executive networks, particularly in the engagement of the DMN. Dysfunction of the major nodes of the DMN (i.e., PCC and mPFC) may be key to understanding deficits of the DMN, its functional dissociation from the SN and CEN, and the subsequent array of cognitive and emotional concussion symptoms in adolescents. Detecting abnormalities in functional connectivity during development may be informative of potential long-term deficits associated with concussion.Acknowledgements
We would like to thank the children and families who dedicated their time and efforts to the collection of the data presented in this study. We would like to acknowledge the nurses, doctors, and clinicians of the McMaster University Children’s Hospital, surrounding Hamilton healthcare centres, and Imaging Research Centre at St. Joseph’s Hospital for supporting this research project. This work was supported by the Canadian Institute of Health Research, awarded to Professor Carol DeMatteo, the Principal Investigator.References
1. Menon, V. (2011). Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci. 15.
2. Menon, V., and Uddin, L.Q. (2010). Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 214, 655–667.
3. Sours, C., Zhuo, J., Janowich, J., Aarabi, B., Shanmuganathan, K., and Gullapalli, R.P. (2013). Default mode network interference in mild traumatic brain injury – A pilot resting state study. Brain Res. 1537, 201–215.
4. Jackson, G.D., Makdissi, M., Pedersen, M., Parker, D.M., Curwood, E.K., Farquharson, S., Connelly, A., Abbott, D.F., and McCrory, P. (2019). Functional brain effects of acute concussion in Australian rules football players. J. Concussion 3, 205970021986120.
5. van der Horn, H.J., Liemburg, E.J., Aleman, A., Spikman, J.M., and van der Naalt, J. (2016). Brain Networks Subserving Emotion Regulation and Adaptation after Mild Traumatic Brain Injury. J. Neurotrauma 33, 1–9.
6. Muller, A.M., and Virji-Babul, N. (2018). Stuck in a State of Inattention? Functional Hyperconnectivity as an Indicator of Disturbed Intrinsic Brain Dynamics in Adolescents With Concussion: A Pilot Study. ASN Neuro 10, 175909141775380.
7. Di Martino, A., Yan, C.-G., Li, Q., Denio, E., Castellanos, F.X., Alaerts, K., Anderson, J.S., Assaf, M., Bookheimer, S.Y., Dapretto, M., Deen, B., Delmonte, S., Dinstein, I., Ertl-Wagner, B., Fair, D.A., Gallagher, L., Kennedy, D.P., Keown, C.L., Keysers, C., Lainhart, J.E., Lord, C., Luna, B., Menon, V., Minshew, N.J., Monk, C.S., Mueller, S., Müller, R.-A., Nebel, M.B., Nigg, J.T., O’Hearn, K., Pelphrey, K.A., Peltier, S.J., Rudie, J.D., Sunaert, S., Thioux, M., Tyszka, J.M., Uddin, L.Q., Verhoeven, J.S., Wenderoth, N., Wiggins, J.L., Mostofsky, S.H., and Milham, M.P. (2014). The autism brain imaging data exchange: towards a large-scale evaluation of the intrinsic brain architecture in autism. Mol. Psychiatry 19, 659–67.
8. Whitfield-Gabrieli, S., and Nieto-Castanon, A. (2012). Conn: A Functional Connectivity Toolbox for Correlated and Anticorrelated Brain Networks. Brain Connect. 2, 125–141.
9. Jafri, M., Pearlson, G., Stevens, M., and Calhoun, V. (2008). A method for functional network connectivity among spatially independent resting-state components in schizophrenia. Neuroimage 39, 1666–1681.