1224
Quantitative functional brain mapping imaging using Arterial Spin Labelling for safe neurosurgery
Giannina Rita Iannotti1,2, Isaure Nadin1, Quentin Tourdot3, Shahan Momjian1, Karl Schaller1, Karl Lovblad2, and Frédéric Grouiller4,5
1Department of Neurosurgery, University Hospital of Geneva, Geneva, Switzerland, 2Department of Radiology and Medical Informatics, University Hospital of Geneva, Geneva, Switzerland, 3Faculty of Pharmacy, University of Montpellier, France, Montpellier, France, 4Laboratory of Behavioral Neurology and Imaging of Cognition, Department of Fundamental Neuroscience, University of Geneva, Geneva, Switzerland, 5Swiss Center for Affective Sciences, University of Geneva, Geneva, Switzerland
1Department of Neurosurgery, University Hospital of Geneva, Geneva, Switzerland, 2Department of Radiology and Medical Informatics, University Hospital of Geneva, Geneva, Switzerland, 3Faculty of Pharmacy, University of Montpellier, France, Montpellier, France, 4Laboratory of Behavioral Neurology and Imaging of Cognition, Department of Fundamental Neuroscience, University of Geneva, Geneva, Switzerland, 5Swiss Center for Affective Sciences, University of Geneva, Geneva, Switzerland
Synopsis
Brain functional mapping is fundamental in pre-surgical workflow to target areas to be preserved. This work aims at validating ASL as alternative to BOLD to map eloquent cortex, by offering a quantitative measure of the perfusion associated to neuronal activity. Thirty healthy subjects underwent TMS motor mapping and executed a clenching-motor-task in a 3T-MR-scanner, while ASL and BOLD volumes were acquired simultaneously. The comparison between the global maxima of BOLD and ASL activations revealed that ASL localizes significantly deeper and more anteriorly than BOLD. ASL global maximum was found closer than BOLD to the most representative point of TMS mapping.
Introduction
Functional Magnetic Resonance Imaging (fMRI) is a widespread technique to non-invasively investigate brain functions. Blood Oxygenation Level Dependent (BOLD) is by far the most used contrast for fMRI1,2. However, BOLD is an indirect measure of neuronal activity3,4,5 and its specificity can be biased by draining veins and by altered neurovascular coupling, typical in neurological patients6,7,8,9.One of the main clinical fMRI application is the pre-surgical mapping of eloquent cortex (language, motor, visual, somatosensory) that should be preserved during neuro-surgery.
Pre-operatively, mainly for motor and language functions, fMRI is corroborated by Transcranial Magnetic Stimulation (TMS) mapping, given its high spatial resolution (0.5-1 mm)10,11,12,13.
Arterial Spin Labelling (ASL) is an additional non-invasive imaging technique which allows for a direct and quantitative measurement of the cerebral blood flow (CBF)14,15. ASL is based on the interleaved acquisition of “labelled” and “control” images, in which the inflowing blood is alternatively magnetically labelled before entering the field-of-view. Functional timecourses of perfusion (fASL) can be extracted by pair-wise subtraction of control and labelled images16. BOLD signal timecourses could be extracted by ASL but the sequence parameters are sub-optimal and can decrease BOLD contrast-to-noise ratio.
Although ASL may locate more directly and more precisely than BOLD, it is still occasionally used for functional brain mapping17,18,19, particularly in the presurgical workflow. Indeed, few studies directly compared fASL and BOLD localizations19,20,21,22,23 and a well-defined post-processing pipeline is not established. Dual-echo pseudo-continuous ASL (DE-pcASL) sequences have been developed to simultaneously acquire BOLD and ASL contrasts with optimal parameters18,24,25.
The present work aims to assess the specificity of fASL by comparing the localization of ASL and BOLD in comparison with TMS in healthy subjects, before the application of fASL in the pre-surgical mapping of patients who are candidate for neurosurgery.
Methods
We recruited thirty healthy volunteers ([18-60] year-old). MRI was performed in a Siemens-TRIO 3T scanner with a 32-channel head-coil. Subjects performed a clenching motor task, for each hand, consisting of 8 active/rest blocks of 35s while BOLD and ASL were simultaneously acquired with a DE-pcASL sequence24 (TR=3500ms, TE1/TE2=10/25ms, label duration=1500ms, PLD=1000ms, FOV=205x205mm, 20 slices of 3mm thickness, in-plane resolution=3.2x3.2mm). The FOV was covering the top-half-brain and the labeling plane was positioned 14cm below the center of image slab perpendicularly to carotids, with the help of a rephased-dephased angiography sequence.Structural imaging included a high-resolution 3D-T1 (Multi-Echo MPRAGE: TR=2530ms, TI=1100ms, TE1-4=1.64/3.5/5.36/7.22ms, 1mm isotropic), SWI and M0.Each subject was enrolled in a second session with TMS (Nextim-NBS4) by following a standard clinical protocol26 to map the motor cortex associated to both hands. Imaging preprocessing and analysis were performed using customized scripts in MATLAB, SPM12 and the opensource toolbox ASLtbx27. Preprocessing included realignment (to the first image), coregistration to the 3D-T1, spatial smoothing using an isotropic Gaussian kernel (FWHM=6 mm). For ASL, labelled and control images were realigned together and pair-wise subtraction between label and control was considered to estimate the perfusion. 3D-T1 was segmented and a brain-mask created. Sixteen subjects were analyzed so far. For each subject, GLM models were built to obtain BOLD and perfusion activation maps associated to each hand. For each subject and each hand the coordinates of the global maxima of BOLD and ASL were collected at individual level. Paired t-tests were performed between the two MRI modalities, in order to detect systematic shift between the localization of BOLD and ASL.Group analysis was conducted after normalization to the MNI template to compare ASL and BOLD activation maps. Finally, localizations of BOLD and ASL were compared with TMS.Results
We were able to localize the primary motor area with both modalities in all subjects (p-FWE<0.05). The activation maps in fASL were better shaped on the known anatomical motor areas, without the extension to the somatosensory cortex (Figure 1).Although a systematic comparison with TMS is still missing, visual inspection clearly showed a better link between fASL and TMS than between BOLD and TMS, in terms of the maximum of activation and size of the functional clusters (Figure 2).Paired-t-test showed significant difference (p<0.001) between ASL and BOLD localization in the z direction (Figure 3C), indicating that fASL systematically localizes deeper than BOLD, along the central sulcus. Along the y direction, fASL had a tendency (p=0.048) to localize more anteriorly than BOLD (11/16 and 10/16 subjects, for right and left hand respectively) (Figure 3B). No significant shift was observed along the x coordinate (Figure 3A). These results were confirmed by the activation map at the group level (Figure 4).Discussion and Conclusion
We demonstrated that fASL is a valid alternative to BOLD in mapping eloquent cortex for presurgical evaluation. Results revealed that fASL localizes significantly deeper motor areas in respect to BOLD. A potential shift between fASL and BOLD in the mesio-lateral and anterior-posterior direction need to be confirmed by considering additional subjects. fASL activations follows the expected anatomical structures better than BOLD, whose main cluster extended over the somatosensory cortex. The systematic comparison of each of these two modalities with TMS, should confirm the better specificity of fASL than BOLD for clinical localization of eloquent cortex to be preserved during surgery.Acknowledgements
This work was supported by the funding of Fondation Louis-Jeantet and Fondation Privé HUG in Geneva, SwitzerlandReferences
- Ogawa, S., et al., Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci U S A, 1992. 89(13): p. 5951-5.
- Kwong, K.K., et al., Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci U S A, 1992. 89(12): p. 5675-9.
- Logothetis, N.K., et al., Neurophysiological investigation of the basis of the fMRI signal. Nature, 2001. 412(6843): p. 150-7.
- Buxton, R.B., E.C. Wong, and L.R. Frank, Dynamics of blood flow and oxygenation changes during brain activation: the balloon model. Magn Reson Med, 1998. 39(6): p. 855-64.
- Friston, K.J., et al., Nonlinear responses in fMRI: the Balloon model, Volterra kernels, and other hemodynamics. Neuroimage, 2000. 12(4): p. 466-77.
- Jacobs, J., et al., Hemodynamic changes preceding the interictal EEG spike in patients with focal epilepsy investigated using simultaneous EEG-fMRI. Neuroimage, 2009. 45(4): p. 1220-31.
- Carusone, L.M., et al., Hemodynamic response changes in cerebrovascular disease: implications for functional MR imaging. AJNR Am J Neuroradiol, 2002. 23(7): p. 1222-8.
- Pittau, F., et al., Negative BOLD response to interictal epileptic discharges in focal epilepsy. Brain Topogr, 2013. 26(4): p. 627-40.
- Agarwal, S., et al., Neurovascular uncoupling in resting state fMRI demonstrated in patients with primary brain gliomas. J Magn Reson Imaging, 2016. 43(3): p. 620-6.
- Picht, T., et al., Presurgical navigated TMS motor cortex mapping improves outcome in glioblastoma surgery: a controlled observational study. J Neurooncol, 2016. 126(3): p. 535-43.
- Frey, D., et al., Navigated transcranial magnetic stimulation improves the treatment outcome in patients with brain tumors in motor eloquent locations. Neuro Oncol, 2014. 16(10): p. 1365-72.
- Sollmann, N., et al., The impact of preoperative language mapping by repetitive navigated transcranial magnetic stimulation on the clinical course of brain tumor patients. BMC Cancer, 2015. 15: p. 261.
- Tarapore, P.E., et al., Language mapping with navigated repetitive TMS: proof of technique and validation. Neuroimage, 2013. 82: p. 260-72.
- Williams, D.S., et al., Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proc Natl Acad Sci U S A, 1992. 89(1): p. 212-6.
- Detre, J.A., et al., Perfusion imaging. Magn Reson Med, 1992. 23(1): p. 37-45.
- Liu, T.T. and E.C. Wong, A signal processing model for arterial spin labeling functional MRI. Neuroimage, 2005. 24(1): p. 207-15.
- Paschoal, A.M., et al., Semantic verbal fluency brain network: delineating a physiological basis for the functional hubs using dual-echo ASL and graph theory approach. J Neural Eng, 2021.18(4).
- Tancredi, F.B., I. Lajoie, and R.D. Hoge, Test-retest reliability of cerebral blood flow and blood oxygenation level-dependent responses to hypercapnia and hyperoxia using dual-echo pseudo-continuous arterial spin labeling and step changes in the fractional composition of inspired gases. J Magn Reson Imaging, 2015. 42(4): p. 1144-57.
- Federspiel, A., et al., Comparison of spatial and temporal pattern for fMRI obtained with BOLD and arterial spin labeling. J Neural Transm (Vienna), 2006. 113(10): p. 1403-15.
- Kallioniemi, E., et al., Localization of cortical primary motor area of the hand using navigated transcranial magnetic stimulation, BOLD and arterial spin labeling fMRI. J Neurosci Methods, 2016. 273: p. 138-148.
- Pimentel, M.A., et al., Localization of the hand motor area by arterial spin labeling and blood oxygen level-dependent functional magnetic resonance imaging. Hum Brain Mapp, 2013. 34(1): p. 96-108.
- Raoult, H., et al., Arterial spin labeling for motor activation mapping at 3T with a 32-channel coil: reproducibility and spatial accuracy in comparison with BOLD fMRI. Neuroimage, 2011. 58(1): p. 157-67.
- Diekhoff, S., et al., Functional localization in the human brain: Gradient-Echo, Spin-Echo, and arterial spin-labeling fMRI compared with neuronavigated TMS. Hum Brain Mapp, 2011. 32(3): p. 341-57.
- Jog, M.A., et al., Developmental trajectories of cerebral blood flow and oxidative metabolism at baseline and during working memory tasks. Neuroimage, 2016. 134: p. 587-96.
- Faraco, C.C., et al., Dual echo vessel-encoded ASL for simultaneous BOLD and CBF reactivity assessment in patients with ischemic cerebrovascular disease. Magn Reson Med, 2015. 73(4): p. 1579-92.
- Krieg, S.M., et al., Protocol for motor and language mapping by navigated TMS in patients and healthy volunteers; workshop report. Acta Neurochir (Wien), 2017. 159(7): p. 1187-1195.
- Wang, Z., et al., Empirical optimization of ASL data analysis using an ASL data processing toolbox: ASLtbx. Magn Reson Imaging, 2008. 26(2): p. 261-9.
Figures

Figure 1: Example of right motor
task. Activations for the right
hand clenching task in subject 3. fASL activation (C )) in respect to BOLD (B))
better follows the profile of the anatomical region known to be associated to
the representation of the right hand (A)).
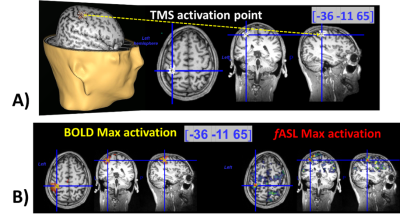
Figure 2. Example of comparison
between fASL, BOLD, TMS in subject 10. In A), 3D and 2D visualization
of the TMS over the left motor area. The yellow arrow and the blue lines
indicate the point where the maximal
amplitude of the evoked motor response of the right thumb was observed. The
coordinates indicated in blue are evaluated in the individual space. By visualizing
the BOLD and fASL activation maps associated to the right hand clenching on the
same coordinates (B)), fASL localizes better than
BOLD (by considering the global maxima of the maps) relatively to TMS
activation.
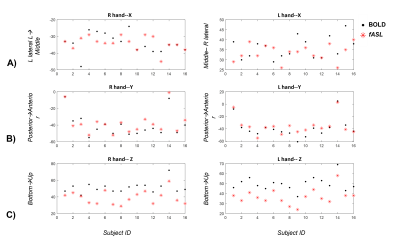
Figure 3: Comparison between the
localization of fASL and BOLD. The coordinates x (lateral-medial),
y( posterior-anterior), z(caudal rostral) are represented for the global maxima
of the BOLD (black dots) and for the global maxima of the fASL (red stars). A significant systematic (p<0.001) shift
is observed for the z coordinate (C )), for both hands, indicating that fASL localizes deeper than BOLD, along the central sulcus. A
trend (p=0.048) of fASL in localizing more
anterior than BOLD can be also observed (B)). No significant or
consistent shift is observed along the x direction(A)).
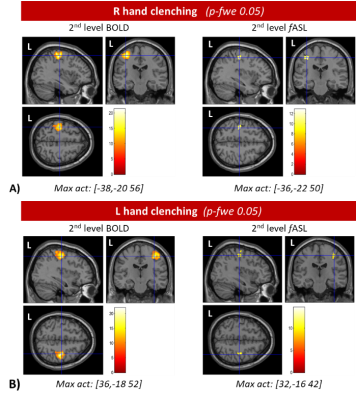
Figure 4: Group analysis. Results of the 2nd
level analysis for right and left hand are visualized for BOLD and fASL,
overlayed with an MNI anatomical template. MNI coordinates are reported below
each picture. Results are visualized at the p-FWE<0.05.
DOI: https://doi.org/10.58530/2022/1224