1220
Novel arterial spin labelling (ASL) brain injury symmetry assessment in retired professional athletes: a preliminary study1School of Biomedical Engineering, McMaster University, Hamilton, ON, Canada, 2Imaging Research Centre, St. Joseph's Healthcare Hamilton, Hamilton, ON, Canada, 3Radiology and Nuclear Medicine, Amsterdam University, Amsterdam, Netherlands, 4Institute of Radiopharmaceutical Cancer Research, Helmholtz-Zentrum Dresden-Rossendorf, Dresden, Germany, 5Ghent Institute for Functional and Metabolic Imaging, Ghent University, Ghent, Belgium, 6Department of Electrical and Computer Engineering, McMaster University, Hamilton, ON, Canada
Synopsis
3D PCASL scans were acquired for seventeen aging, retired professional football players with a history of head traumas. Left, right and bilateral CBF and ASL spatial coefficient of variation (sCoV) values were examined for twelve concussion-related ROIs. A Z-scoring approach was applied, with outliers defined as mild, moderate, or severe injury burden (IB). An IB symmetry index was also calculated. Outliers were detected in all 12 ROIs, and the anterior parahippocampal gyrus and inferior frontal gyrus pars opercularis had the highest CBF and ASL sCoV IB, respectively. IB was not biased towards the left or right hemisphere.
Introduction
High contact sport athletes can sustain multiple concussions and countless sub-concussive blows over the course of their career, potentially accumulating to neurological damage and even dementia.1 Arterial spin labelling (ASL), which allows for the non-invasive measurement of cerebral perfusion, may be a valuable diagnostic technique to study this damage.1 Previously,2 we explored the association of ASL spatial coefficient of variation (sCoV) with the aim of identifying atypical brain regions related to concussive injuries. Our current work adds to this by performing grey matter normalization to improve inter-subject comparability and including cerebral blood flow (CBF) as a more standard metric relative to the novel ASL sCoV values. Thus, this current study used ASL and a Z-scoring approach to quantify long term CBF and ASL sCoV alterations, indicative of focal brain damage, and hemispheric microvascular symmetry in retired Canadian Football League (rCFL) players. We hypothesized that CBF and ASL sCoV would be symmetric and have regional dysregulation due to the subjects’ history of repetitive head trauma.Methods
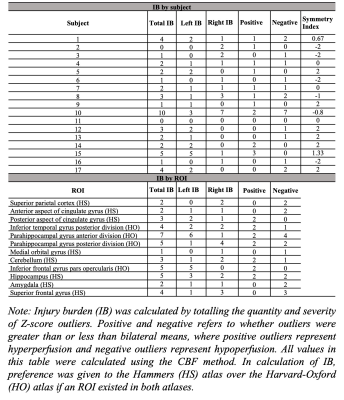
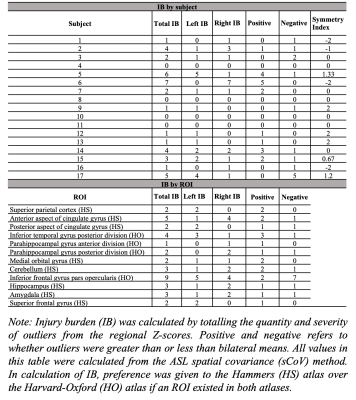
Seventeen rCFL players (all male, aged 58±6.15y) were scanned using a 3T GE Discovery MR750 MRI and a 32-channel head coil. 3D T1-weighted fSPGR (TR/TE/flip=11.34/4.25ms/12°, 256x256 matrix with 1mm slice thickness, 1mm isotropic) and 3D pseudo-continuous ASL (PCASL) (PLD=1525ms, labeling duration=1450ms, TR/TE=4886/10.528ms, NSA=4, background-suppressed, separate M0 scan) scans were acquired. The PCASL scans were processed using ExploreASL to perform brain segmentation, motion correction, spatial normalization to MNI152 T1 1.5mm space, partial volume correction, and regional quantification of CBF and ASL sCoV.3 Twelve regions-of-interest (ROIs) used in previous concussion and dementia studies 4-8 were selected from the Harvard-Oxford (HO)9 and Hammers (HS)10 atlases (Figure 1). ROI CBF and ASL sCoV data were analyzed bilaterally as well as separately for the left and right hemispheres. Prior to Z-scoring, ROIs that failed Shapiro-Wilk normality testing or had insufficient data (>2 data points missing) were excluded. Bilateral, left, and right ROI values were normalized to each subject’s bilateral, left- and right-hemisphere total grey matter (GM) CBF and ASL sCoV, respectively. A Z-scoring approach was applied with the bilateral CBF and ASL sCoV values as the comparative metric. Z-score outliers that fell 2, 3, or 4 standard deviations from the bilateral ROI means were classified as a mild (1), moderate (2), or severe (3) injury burden (IB). A subject-specific IB symmetry index was calculated as: (L-R)/(0.5(L+R)). Normality, multiple linear regression, and correlation tests were performed with a Bonferroni correction. Age, expected type of positional head impact(s), and career length were used as covariates.Results
Outliers, falling 2, 3, or 4 standard deviations from the bilateral group means, were detected in all 12 ROIs investigated (CBF IB=2.53±2.38, ASL sCoV IB=2.24±2.22). From the CBF analysis, the parahippocampal gyrus (anterior)(IB=7), parahippocampal gyrus (posterior)(IB=5), hippocampus (IB=5), and inferior frontal gyrus pars opercularis (IB=5) had the highest IBs (Table 1). From the ASL sCoV analysis, the inferior frontal gyrus pars opercularis (IB=9), cingulate gyrus (anterior)(IB=5) and inferior temporal gyrus posterior division (IB=4) had the highest IBs (Table 2). Groupwise, IB was symmetric (CBF: IB index=0.1375±1.62, left=23, right=20; ASL sCoV: IB index=0.13±1.33, left=19, right=19). There were also more negative outliers (negative=20; positive=14). From the CBF IB data, only right IB and negative Z-scores had a significant association in the linear model (estimate=0.830, p=0.00135). From the ASL sCoV data, there were no significant associations. Total IB and symmetry index score calculated from both CBF and ASL sCoV data also had no significant correlations with demographic factors.Discussion
IB was symmetric when calculated for the entire group, but IB was often unilateral for each subject. In agreement with literature, the parahippocampal gyri and hippocampus may be especially vulnerable to injury.4,5,7,8 These regions are vitally responsible for memory, emotion, and cognitive control.11,12 Furthermore, the left inferior frontal gyrus pars opercularis partially comprises Broca’s area to be integral to language production,13 and the right side has been linked to impulse control and depression.14,15 Finally, the hypo- and hyperperfusion CBF results were inconclusive and subject-specific as has been seen in other studies.1 It should be noted that the IB scores calculated for most subjects and ROIs in this current study differed from our previous findings.2 The cingulate gyrus and inferior temporal gyrus posterior division were also previously identified as commonly atypical ROIs from ASL sCoV analyses, however the inferior frontal gyrus pars opercularis was not especially atypical prior to GM CBF normalization.2 Thus, this current study may have provided more robust results through its inclusion of CBF as a more reliable metric and due to more thorough data processing via normalization.Conclusion
Furthermore, microvascular cerebral dysregulation may be present decades after the last concussion. The inferior frontal gyrus pars opercularis was commonly identified as injured for both CBF and ASL sCoV, however future studies should be performed in this field to validate our findings. Further studies may analyze concussion CBF and ASL sCoV in relation to DTI and fMRI.Acknowledgements
No acknowledgement found.References
1. Hart J, Kraut MA, Womack KB, et al. Neuroimaging of Cognitive Dysfunction and Depression in Aging Retired National Football League Players: A Cross-sectional Study. JAMA Neurol. 2013;70(3):326-335.
2. Danielli E, Padrela B, Doughty M, et al. Assessment of cerebral perfusion symmetry in retired Canadian Football League players. ESMRMB. 2021;S2.P10. (Poster). Virtual.
3. Mutsaerts HJMM, Petr J, Groot P, et al. ExploreASL: An image processing pipeline for multi-center ASL perfusion MRI studies. NeuroImage. 2020;219:117031.
4. Churchill N, Hutchinson M, Graham SJ, et al. Symptom correlates of cerebral blood flow following acute concussion. NeuroImage Clin. 2017;16:234-239.
5. Cassoudesalle H, Petit A, Chanraud S, et al. Changes in resting-state functional brain connectivity associated with head impacts over one men’s semi-professional soccer season. J Neurosci Res. 2020;00:1-9.
6. Clark AL, Weigand AJ, Bangen KJ, et al. Repetitive mTBI is associated with age-related reductions in cerebral blood flow but not cortical thickness. J Cereb Blood Flow Metab. 2021;41(2):431-444.
7. Bangen KJ, Nation DA, Clark LR, et al. Interactive effects of vascular risk burden and advanced age on cerebral blood flow. Front Aging Neurosci. 2014;6:159.
8. Dickerson BC, Bakkour A, Salat DH, et al. The cortical signature of Alzheimer’s Disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex. 2009;19(3):497-510.
9. Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31(3):968-980.
10. Hammers A, Allom R, Koepp MJ, et al. Three‐dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Hum Brain Mapp. 2003;19:224-247.
11. Sharma N, Murari G, Vandermorris S, et al. Functional connectivity between the posterior default mode network and parahippocampal gyrus is disrupted in older adults with subjective cognitive decline and correlates with subjective memory ability. J Alzhiemer’s Dis. 2021;82(1):435-445.
12. Wang Z, Dong H, Du X, et al. Decreased effective connection from the parahippocampal gyrus to the prefrontal cortex in Internet gaming disorder: a MVPA and spDCM study. J Behav Addict. 2020;9(1):105-115.
13. Amunts K and Zilles K. Architecture and organizational principles of Broca’s region. Trends Cogn Sci. 2012;16(8):418-426.
14. Du J, Rolls ET, Cheng W, et al. Functional connectivity of the orbitofrontal cortex, anterior cingulate cortex, and inferior frontal gyrus in humans. Cortex. 2020;123:185-199.
15. Rolls ET, Cheng W, Du J, et al. Functional connectivity of the right inferior frontal gyrus and orbitofrontal cortex in depression. Soc Cogn Affect Neurosci. 2020;15(1):75-86.
Figures