1216
Non-invasive assessment of blood-brain barrier permeability in multiple sclerosis patients by arterial spin labeling.
Andre Monteiro Paschoal1, Mateus Boaventura1, Leonie Petitclerc2, Matthias J.P. van Osch2, Maria Concepcion Garcia Otaduy1, Samira Luisa Apostolos-Pereira3, Dagoberto Callegaro3, and Carolina de Medeiros Rimkus1
1Institute of Radiology and Department of Radiology and Oncology, University of Sao Paulo, Sao Paulo, Brazil, 2C.J. Gorter Center for high field MRI, Department of Radiology, Leiden University Medical Center, Leiden, Netherlands, 3Departamento de Neurologia, Hospital das Clinicas da Faculdade de Medicina, University of Sao Paulo, Sao Paulo, Brazil
1Institute of Radiology and Department of Radiology and Oncology, University of Sao Paulo, Sao Paulo, Brazil, 2C.J. Gorter Center for high field MRI, Department of Radiology, Leiden University Medical Center, Leiden, Netherlands, 3Departamento de Neurologia, Hospital das Clinicas da Faculdade de Medicina, University of Sao Paulo, Sao Paulo, Brazil
Synopsis
Multiple sclerosis is a demyelinating disease affecting the central nervous system. Although MS lesions can be identified by conventional MRI, the evaluation of active lesions in normal appearing gray and white matter requires measures of blood-brain barrier permeability (BBB), which is commonly assessed through the dynamic contrast enhanced (DCE) acquisition. When repeated scans are needed in the MS patients follow-up, repeated gadolinium injection is not recommended. This study successfully showed the feasibility of advanced arterial spin labeling sequences as an alternative to evaluate BBB permeability, whose results of water-exchange times across the BBB were in agreement with DCE measurements.
Introduction
Multiple sclerosis (MS) is the most inflammatory demyelinating disease that affects the central nervous system (CNS), characterized by focal lesions and diffuse damage of both white and gray matter1. MS pathophysiology is associated with blood-brain barrier (BBB) permeability deregulation, allowing leakage of auto-reactive T-cells to perivascular compartments2. Although macroscopic MS lesions are easily identified as hyperintensities in T2w or FLAIR, it is not possible to differentiate active from inactive lesions solely based on these images. Inflammatory and microstructural changes in MS go far beyond focal lesions, affecting normal appearing white (NAWM) and gray matter (NAGM)2. Recent evidence has shown signs of latent inflammation in chronic lesions, the so-called smoldering lesions2,3. In parallel, histopathological studies have reported BBB abnormalities in inactive MS lesions and in NAWM4.Common strategies to evaluate BBB damage in NAWM of MS patients are based on post-contrast T1w-MRI or dynamic contrast-enhanced (DCE) MRI, from which perfusion and permeability information is obtained5. Since the chronic nature of MS, repeated MRI scans are necessary, including repeated injection of gadolinium-based contrast agents, which is unwanted due to concerns regarding gadolinium retention. A novel non-invasive alternative to contrast-enhanced methods has recently been proposed employing advanced arterial spin labeling (ASL) sequences6,7, allowing the quantification of water-exchange times across the BBB, beyond classical outputs of cerebral blood flow (CBF) and arterial transit time (ATT).
In this study, we employed Hadamard-encoded pseudo-continuous ASL (pCASL) to acquire images after seven post-labeling delays (PLDs) combined with a multi-echo 3D-GRASE readout to assess BBB permeability in MS patients and compare it to the results of DCE and pre- and post-contrast T1w, T2w and FLAIR.
Methods
Three MS patients (one female, 37.33-13.65 years) were scanned on a Philips Achieva 3T MRI scanner in agreement with local IRB conditions. The total acquisition protocol consisted of pre- and post-contrast T1w, T2w, FLAIR, ASL and DCE (Figure 1). The ASL protocol included seven PLDs (170-1870ms) after eight Hadamard-blocks with labeling duration of 1800, 800, 400, 2x150 and 2x100 ms to capture both angiographic and perfusion phases and eight echo times (13-393 ms) to evaluate the T2-signal decay and separate the contributions of GM and blood.DCE post-processing was done using the PkModeling extension of 3D-Slicer to obtain Ktrans maps. ASL post-processing was performed with in-house scripts in Julia Language8 using a bi-exponential model in two stages: First, CBF and ATT were obtained by fitting the shortest echo signal to the ASL-Buxton model9; and second by fitting the multi-echo signal decay considering literature values of T2 for brain and blood, providing the exchange time between blood and GM (Tblgm)10.
All images were transformed to ASL space using FSL-FLIRT11. Data analysis was based on the ROIs of inflammatory lesions, defined by FLAIR hyperintensities, and NAWM with active MS identified as CE voxels by DCE-images. Quantitative plots were made in R12.
Results
For the ROIs of inflammatory FLAIR hyperintensities, lesions were mostly DCE/postGD-T1w non-enhancing (NE), barring a few voxels localized deep within the lesions, manifesting as contrast-enhanced (CE), as illustrated in Figure 2. For NAWM ROI, the analysis revealed contrast-enhancing regions in combination with increased CBF, Tblgm and Ktrans, but no signs of inflammation in FLAIR (Figure 3). The quantitative measurements of healthy and BBB damaged NAWM are plotted in Figure 4.Discussion
An important disease activity biomarker in MS patients refers to BBB breakdown, since the leakage of auto-aggressive T-cells advance neuronal demyelination2, typically visualized as FLAIR hyperintensities (Fig.2). However, T2-FLAIR hyperintensities are non-specific to underlie pathological processes, and might correspond to active demyelination/inflammation, edema, gliosis or remyelination areas. The BBB permeability evaluation might help to understand the dynamic of pathological processes in MS. Our study revealed that most of T2-FLAIR hyperintense lesions did not show contrast-enhancement, which is the most conventional clinical practice to measure active inflammation. This pattern was replicated by analyzing ASL T2blgm. In contrast, deep inside some macroscopic lesions, we observed signs of BBB damage in both ASL-Tblgm and DCE-Ktrans, suggesting BBB dysfunction, which might be a sign of silent inflammatory activity, not detectable by T1-Gd images.ASL measures of BBB dysfunction in NAWM were in agreement to classical permeability measurements of DCE and postGd-T1w, with the pattern of CBF and T2blgm values (Fig.4) meeting the NAWM contrast-enhancement foci with increased Ktrans values. The need for repeated BBB permeability measures in the follow-up of MS patients strengthens the necessity of contrast-free approaches, with ASL being a promising alternative. Furthermore, as ASL measures are based on water molecules, it may be more sensitive to detect subtle BBB deregulation than Gd-leakage, but maybe less spatially specific as the water exchange happens between multiple compartments.
Conclusion
This study showed the ASL utility in evaluating perfusion and BBB-permeability in MS patients, providing two-fold contributions. First, since BBB damage in MS lesions is not as dramatic as in e.g. glioma, it can add on as a methodological proof-of-concept regarding the feasibility of ASL for BBB permeability assessment in clinical routine. Second, by providing results comparable to DCE, ASL is an interesting non-invasive alternative to investigate BBB damages in NAWM.Acknowledgements
Fundação de Amparo à Pesquisa do estado de Sao Paulo (FAPESP), grant number: 2020/13586-7References
1. Cotsapas, et. al. Multiple sclerosis. 1st ed. Vol. 148, Handbook of Clinical Neurology. Elsevier B.V.; 2018. 723–730 p. Available from: http://dx.doi.org/10.1016/B978-0-444-64076-5.00046-62. Cramer, et. al. Abnormal blood-brain barrier permeability in normal appearing white matter in multiple sclerosis investigated by MRI. NeuroImage Clin [Internet]. 2014;4:182–9. Available from: http://dx.doi.org/10.1016/j.nicl.2013.12.001
3. Ingrisch, et. al. Quantification of Perfusion and Permeability in Multiple Sclerosis. Invest Radiol. 2012;47(4):252–8.
4. McQuaid, et. al. The effects of blood-brain barrier disruption on glial cell function in multiple sclerosis. Biochem Soc Trans. 2009;37(1):329–31.
5. Tofts PS, Parker GJM. DCE-MRI: Acquisition and analysis techniques. Clin Perfus MRI Tech Appl. 2010;9781107013:58–74.
6. Gregori, et. al. T2-based arterial spin labeling measurements of blood to tissue water transfer in human brain. J Magn Reson Imaging. 2013;37(2):332–42.
7. Petitclerc, et. al. Combining T 2 measurements and crusher gradients into a single ASL sequence for comparison of the measurement of water transport across the blood – brain barrier. 2020;(November):1–12.
8. https://julialang.org/
9. Buxton, et. al. A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magn Reson Med. 1998;40(3):383–96. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9727941
10. Petitclerc, et. al. Blood-CSF Barrier Imaging in the Human Brain with Arterial Spin Labeling. Proc 21th Sci Meet Int Soc Magn Reson Med 2021. 2021;4–6.
11. https://fsl.fmrib.ox.ac.uk/fsl/fslwiki
12. https://www.r-project.org/
Figures
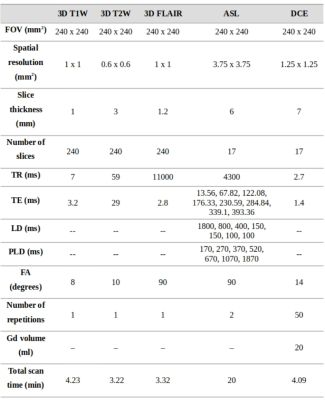
Figure 1: Detailed parameters of the MRI acquisition protocol.
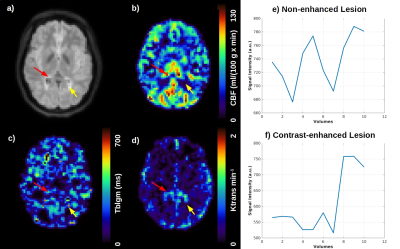
Figure 2: a) Inflammatory MS lesions identified as bright regions in FLAIR-MRI and the perfusion-related parameter: b) CBF in ml/(100 g * min); c) Exchange time across the BBB (Tblgm) measured by ASL in ms and d) Ktrans map measured in min-1, obtained through the contrast-enhanced DCE time-series shown in e) for a non-enhanced lesion (yellow arrow) and f) contrast-enhanced lesion (red arrow).

Figure 3: Normal Appearing White Matter (NAWM) analysis. a) FLAIR images showed no inflammatory MS lesions in the regions pointed by the red and yellow arrows and the perfusion-related parameter: b) CBF in ml/(100 g * min); c) Exchange time across the BBB (Tblgm) measured by ASL in ms and d) Ktrans map measured in min-1, obtained through the contrast-enhanced DCE time-series shown in e) NAWM regions with no BBB damages and f) NAWM regions with BBB damage.
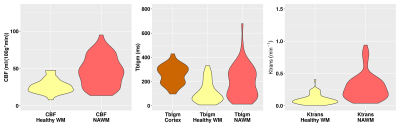
Figure 4: Violin plots of the quantitative measurements of CBF in ml/(100 g * min) and Ktrans in min-1 for a healthy WM region and for a damaged BBB region in NAWM and Tblgm measured in ms in GM cortex, healthy WM and BBB damaged NAWM regions.
DOI: https://doi.org/10.58530/2022/1216