1213
Ovariectomized Female Rats Suffer Hippocampal Neuroinflammation and the Blood-Brain Barrier Disruption Easier than Male and Intact Female Rats following Prolonged Exposure of a Hypobaric and Hypoxic Environment at High Altitude1Department of Radiology, West China Hospital of Sichuan University, Chengdu, China
Synopsis
Ovariectomized Female Rats Suffer Hippocampal Neuroinflammation and the Blood-Brain Barrier Disruption Easier than Male and Intact Female Rats following Prolonged Exposure of a Hypobaric and Hypoxic Environment at High Altitude
It is well-known that both the estrogen and testosterone have neuroprotective effects in neuronal system [1,2]. Therefore, in this study, we hypothesized that OVX female animals can be more prone to hippocampal damage than intact females and males due to low levels of neuroinflammation and BBB permeability. Thus, the aim of this study to investigate the gender difference in hypobaric hypoxia induced neuroinflammation and blood-brain barrier changes by establishing a real plateau environment-induced hypobaric hypoxia model with male, and female integral and castrated rats.
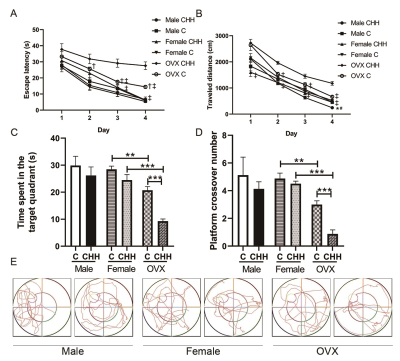
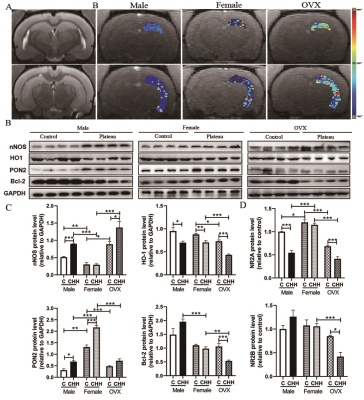
Objective: The present study aimed at investigating the role of sex in hypobaric hypoxia induced neuroinflammation and blood-brain barrier changes in adult male, female, and ovariectomized (OVX) female rats. Materials and Methods: Male, intact female, and ovariectomized (OVX) female rats were randomly assigned to chronic hypobaric hypoxia (CHH) group and control (C) group. The CHH group was transported from Chengdu to the Qinghai-Tibet Plateau (4250 m above sea level (a.s.l.)) and was then fed for 1 month, while the C group was reared in Chengdu (500 m a.s.l.). One month later, dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) at a 7.0T preclinical MR scanner (Bruker, Ettlingen, Germany) was used to evaluate BBB permeability in the hippocampus in vivo. In addition, biochemical analysis, behavioral assessment, histological, and molecular examinations were performed on each group. Results: After 1 month of exposure to plateau hypoxia, OVX female animals had a learning deficit in the Morris water maze, while male and intact females did not show this deficit. DCE-MRI analysis of the same animals indicated higher levels of BBB permeability of OVX female animals than intact females or males. Biochemical methods showed that the contents of MDA, and the contents of IL-6, IL-1β, and TNF-α in hippocampus were increased, while the activities of SOD, GSH, GSH-Px, and CAT decreased in OVX female animals. Immunohistochemical staining results showed that increased activation of microglia in the hippocampus. At the molecular level, hypobaric hypoxia promoted the activation of nNOS, and decreased the levels of HO-1 and paraoxonase 2 (PON2) expression in the hippocampal region caused by deficiency of estrogen. Additionally, we found that hypobaric hypoxia decreased the gene expression of the NR2A and NR2B subunit of N-methyl-D-aspartate (NMDA) receptors in the hippocampus of OVX female rats. Conclusion: A lack of ovarian function may increase neuroinflammation and blood-brain barrier permeability at altitude.
Discussion and conclusion
Under hypobaric hypoxia, OVX female rats exhibit a higher incidence of neuroinflammation and blood-brain barrier disruption than intact female and male rats in our study. Further pathology and molecular examination confirmed this finding. Thus, the sex hormone estrogen is thought to play a critical role in preventing neuroinflammation and blood-brain barrier disruption encountering chronic hypobaric hypoxia.
Our data also indicate that plateau hypoxia exposure caused oxidative stress and neuroinflammation in male and female rats that extended in hippocampus tissue but was more pronounced in the male and OVX female rats than intact female brain. Therefore, the OVX female rats may be more prone to plateau hypoxia induced hippocampal damages than male and intact female rats due to low levels of paraoxonase 2 (PON2) expression, intracellular antioxidants, and anti-inflammatory enzymes.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (no. 81930046, 81771800, and 81829003).References
1. Lara A, Esperante I, Meyer M, Liere P, Di Giorgio N, Schumacher M, Guennoun R, Gargiulo-Monachelli G, De Nicola AF, Gonzalez Deniselle MC: Neuroprotective Effects of Testosterone in Male Wobbler Mouse, a Model of Amyotrophic Lateral Sclerosis. Molecular neurobiology 2021, 58(5):2088-2106.
2. Yang Y, Zhao L, Li N, Dai C, Yin N, Chu Z, Duan X, Niu X, Yan P, Lv P. Estrogen Exerts Neuroprotective Effects in Vascular Dementia Rats by Suppressing Autophagy and Activating the Wnt/β-Catenin Signaling Pathway. Neurochem Res. 2020 Sep;45(9):2100-2112. doi: 10.1007/s11064-020-03072-5
Figures