1210
Detection of enhanced cerebrospinal fluid transport during dexmedetomidine anesthesia in the rat using diffusion, T2 and morphometric MRI1Biomedical Engineering, University of Arizona, Tucson, AZ, United States, 2NICHD/NIH, Bethesda, MD, United States
Synopsis
The enhancement of CSF transport in a robust experimental model was measurable using in-vivo quantitative MRI.TBM and voxelwise T2 mapping analysis were both able to changes in regions that are known to be associated with CSF transport based on contrast-enhancement studies. These two methods do not require contrast and if validated could provide a non-contrast outcome metric for health of the CSF transport system. Diffusion MRI outcomes suggest that it may be possible to sensitize the diffusion MRI paradigm to CSF transport and this is an area for future research.
Introduction
The active transport of cerebrospinal fluid (CSF) in the brain may be important for waste clearance and a site of dysfunction in brain disease and injury(1–3). Pre-clinical MRI studies in rodents have been used to better understand the basic physiologic aspects of this system using both contrast (4,5) and non-contrast methods, including by controlling CSF transport states using isoflurane and dexmedetomidine(6,7). While these studies have begun to elucidate fundamental aspects of CSF transport, considerable gaps in knowledge remain about basic physiology of CSF transport and translational MRI markers for this phenomenon are quite nascent.The objective of the present study was to identify quantitative MRI techniques able to detect CSF transport enhancement in anesthetized rats. Tensor-based morphometry (TBM) was employed to evaluate morphologic alterations and T2 mapping was used to evaluate changes in the biochemical environment by voxelwise analysis. Single cortical slice mapping was used to visualize arterioles and venules and a dual-tensor fitting of diffusion MRI data was used to compare diffusivity and volume fractions between anesthesia conditions. The observations gained from these studies advance the fundamental understanding of CSF transport in the brain.
Methods
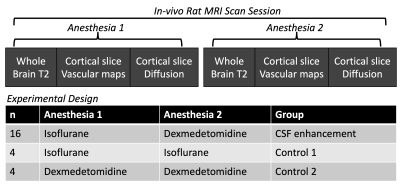
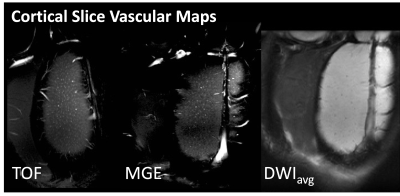
In-vivo MRI scans were performed for 8 female Sprague Dawley rats and each rat was imaged 3 times including twice in the CSF enhancement paradigm and once in a control paradigm. During the CSF enhancement paradigm rats were imaged under inhaled isoflurane anesthesia and then the anesthesia was changed to intravenous dexmedetomidine and the scans were collected again. For the control paradigms imaging was performed sequentially under only isoflurane or dexmedetomidine. These paradigms are shown in figure 1.A Bruker 7T pre-clinical MRI scanner was used to collect whole brain multi-echo T2 MRI scans. Single slice imaging within the cortex included time-of-flight (TOF) angiography and multi-gradient echo (MGE) MRI to generate vascular maps of the penetrating arteriole and venules(8). In the same cortical plane, diffusion MRI was performed using 2D EPI with b=400,600 and 800 s/mm2 with 32 directions each. Fitting of the diffusion tensor for calculation of Trace and also dual-tensor fitting for calculation of free water volume fraction (VF) was performed using TORTOISE (9). ANTs Affine and SyN registration(10,11) was used to register within-session brain volumes for the generation of the TBM LogJ maps(12) – which indicate local volume changes between anesthesia conditions. ANTs tools were also used to register all T2 and LogJ maps into a common space for the generation of average maps for the ratio change in T2 or LogJ across the study.
Results
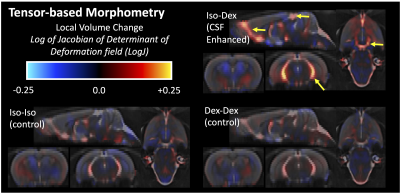
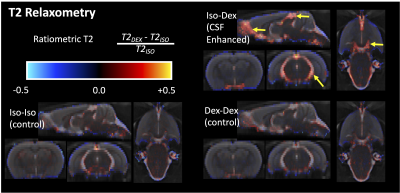
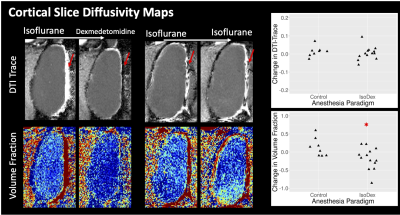
TBM showed striking local volume increases in the CSF spaces for the CSF enhanced group (figure 2) and T2 differences were greatest in these regions as well (figure 3), especially at the boundaries between CSF and the brain. There were no changes in parenchymal T2, although LogJ increases and decreases were evident. The most prominent observation in the diffusion cortical slice mas was the loss of midline diffusivity when anesthesia was switched from isoflurane to dexmedetomidine (red arrows, figure 5) and ROI analyses in these maps revealed a statistically significant (p=0.02) decrease in VF for the CSF enhanced condition compared with controls, but no significant difference for Trace.Discussion
Several key findings in the present study advance MRI measurements of CSF transport in the brain. Voxelwise mapping of LogJ values are consistent with segmentation-based findings(6) and with voxel-based morphometry analysis (7) although appear to be more expansive in mapping regions with increased volume and resemble the maps generated from contrast-based MRI methods. This improvement is likely from the direct nature of the TBM methods to characterize non-linear deformations on a voxelwise basis. T2 values were notably increased in ventricular regions of known CSF flow and completely unchanged in the brain itself, although the source for increased T2 is not immediately clear. If this pattern is associated with CSF transport alterations however, it could be a potentially useful non-contrast method to assess the health of this system.Diffusion MRI is an attractive technique for characterizing CSF transport, but it is quite challenging to sensitize the diffusion experiment to the assumed flow rates for CSF transport. For example, the complete loss of diffusivity at the midline during dexmedetomidine could be related to increased bulk flow, which cannot contribute to diffusivity measurement. The decrease in free water volume fraction in the cortical parenchyma with dexmedetomidine was somewhat unexpected given the increase in extracellular space that has been observed with activation of this system, but additional studies will be necessary to know the physiologic source for the volume fraction finding.
Conclusions
The enhancement of CSF transport in a robust experimental model was measurable using in-vivo quantitative MRI.TBM and voxelwise T2 mapping analysis were both able to changes in regions that are known to be associated with CSF transport based on contrast-enhancement studies. These two methods do not require contrast and if validated could provide a non-contrast outcome metric for health of the CSF transport system. Diffusion MRI outcomes suggest that it may be possible to sensitize the diffusion MRI paradigm to CSF transport and this is an area for future research.Acknowledgements
All imaging was performed in the UA translational bioimaging resource (TBIR) and made possible by the NIH small instrumentation grant: S10 OD025016. The authors would like to thank High Performance Computing (HPC) resource for computing infrastructure.References
1. Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012 Aug 15;4(147).
2. Iliff JJ, Chen MJ, Plog BA, Zeppenfeld DM, Soltero M, Yang L, et al. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci. 2014;34(49):16180–93.
3. Ratner V, Gao Y, Lee H, Elkin R, Nedergaard M, Benveniste H, et al. Cerebrospinal and interstitial fluid transport via the glymphatic pathway modeled by optimal mass transport. Neuroimage. 2017/03/23. 2017;152:530–7.
4. Iliff JJ, Lee H, Yu M, Feng T, Logan J, Nedergaard M, et al. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest. 2013 Mar 1;123(3):1299–309.
5. Lee H, Mortensen K, Sanggaard S, Koch P, Brunner H, Quistorff B, et al. Quantitative Gd-DOTA uptake from cerebrospinal fluid into rat brain using 3D VFA-SPGR at 9.4T. Magn Reson Med. 2017/06/20. 2017;
6. Benveniste H, Lee H, Ding F, Sun Q, Al-Bizri E, Makaryus R, et al. Anesthesia with Dexmedetomidine and Low-dose Isoflurane Increases Solute Transport via the Glymphatic Pathway in Rat Brain When Compared with High-dose Isoflurane. Anesthesiology. 2017/09/25. 2017;127(6):976–88.
7. Ozturk BO, Monte B, Koundal S, Dai F, Benveniste H, Lee H. Disparate volumetric fluid shifts across cerebral tissue compartments with two different anesthetics. Fluids Barriers CNS. 2021 Dec 1;18(1).
8. Yu X, He Y, Wang M, Merkle H, Dodd SJ, Silva AC, et al. Sensory and optogenetically driven single-vessel fMRI. Nat Methods. 2016 Mar 30;13(4):337–40.
9. Irfanoglu MO, Nayak A, Jenkins J, Pierpaoli C. TORTOISE v3: Improvements and New Features of the NIH Di. In: 25th Annual Meeting of the International Society fro Magnetic Resonance in Medicine. Honolulu, HI; 2017.
10. Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. 2008;12(1):26–41.
11. Avants BB, Tustison NJ, Stauffer M, Song G, Wu B, Gee JC. The Insight ToolKit image registration framework. Front Neuroinform. 2014;8:44.
12. Ashburner J, Friston K. Morphometry. Morphometry. 2003;
Figures