1201
Continuous Perfusion Measurement With Singled Sided Low Field MR1The University of Auckland, Auckland, New Zealand, 2Victoria University of Wellington, Wellington, New Zealand, 3University of Otago, Wellington, New Zealand
Synopsis
In this study we focus on exploring the use of a single sided low-field MR systems to continuously measure perfusion. For this purpose we developed a contrast-free measurement technique - tentatively named Inhomogeneous Flow-sensitive Alternating Inversion Recovery (IFAIR). This new technique, adapted from the FAIR ASL protocol, was designed to be suitable for inhomogeneous field conditions and to function without pulsed gradient fields. The IFAIR signal response displayed a consistent proportional relationship to flow velocity, with potential to allow for quantitative flow-dependent measurements.
Introduction
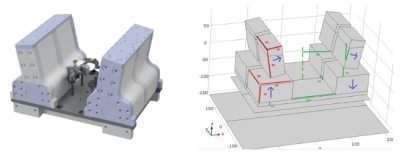
A novel 9 MHz permanent magnet MR system was the core instrument of this project. The system possesses an inhomogeneous B0 field, with a relatively homogeneous sweet spot at the centre with a volume of ~5c (Figure 1). This prototype system was designed to enable low cost, portable MR-based assessment of brain health, such as the diagnosis and active monitoring of strokes. Blood perfusion is one of the fundamental parameters of tissue health and function. And clinically used to assess the severity of strokes, tumours, and other diseases. The device can measure perfusion using standard gadolinium MRI contrast agents. However, this is impractical for continuous monitoring purposes, so a novel spin tagging technique for non-invasively measuring tissue perfusion was developed. Arterial Spin Labelling (ASL) is a widely used exogenous tracer based MRI perfusion measurement technique. These sequences utilize the blood within the body as a temporary tracer, removing the need for the contrast agent injection. They are well suited to situations where contrast agent administration might be contraindicated (e.g. patients with impaired renal function) or for continuous monitoring applications, where repeated administration of a contrast agent is impractical.Methods
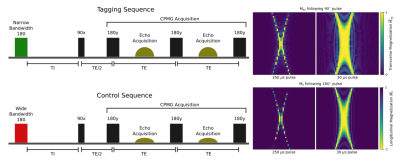
A custom-built magnet controlled by a compact Matipo spectrometer (Resonint Ltd. Wellington) was used for development and testing purposes (Figure 1). A custom-designed flow-phantom consisting of a porous foam inset into a plastic tube was used in a flow circuit running through the 9 MHz system. A Minipuls3 (Gilson Middleton, WI USA) peristaltic pump was used to drive the ASL flow circuit, flow rates were manually calibrated. Two subtly different pulse sequences were defined (Figure 2). The tagging sequence used a consistent narrow bandwidth for all pulses, including the initial inversion. The control sequence was identical, except the bandwidth of the initial inversion was modified, using a shorter and stronger RF pulse. The CPMG echoes were summed to improve the signal-to-noise ratio. Figure 2 shows the structure and relative timing of this pulse sequence. Bloch equation simulations and experimental methods were used to verify the validity of our approach to measure low flow rates, mimicking tissue perfusion.Results
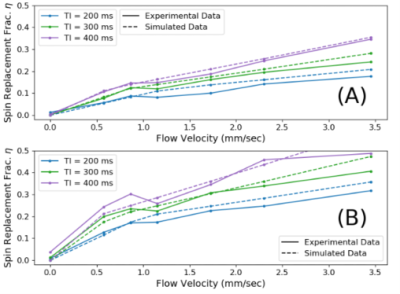
IFAIR protocol was able to detect flow velocity by analysing the difference in signal response between the flow-sensitive tagging sequence and the flow-insensitive control sequence. However, due to the inhomogeneous field, the comparison between the affected volumes of the control and tagging required more careful consideration. Experimental and simulated data shows a good correlation across a range of flow velocities between 0 and 3.5 mm/sec (Figure 3).Conclusion
This project has shown the viability of a contrast-free method for measuring perfusion with low-field MR. Measurement techniques were designed to suit the limitations and strengths of the prototype low-field system and were demonstrated to produce perfusion-dependent results. Combined with the unique advantages of the low-field prototype system, the IFAIR protocol has the potential to allow for semi-continuous acute-stage monitoring of the recovery of perfusion following a stroke, through a portable device. No currently available technology can be feasibly applied to this role.Acknowledgements
This project is funded in part by the New Zealand Ministry of Business Innovation and EmploymentReferences
1. Thomas D. et.al. "Single-sided magnet system for relaxometry and diffusion measurement" (ISMRM 2021)
2. Paul T. Callaghan. "Translational Dynamics and Magnetic Resonance: Principles of Pulsed Gradient Spin Echo NMR." (Oxford University Press 2011)
3. Casanova, F., Perlo, J. & Blümich, B. "Single-Sided NMR." (Springer, 2011)
4. David C. Alsop et al. “Recommended Implementation of Arterial Spin Labeled Perfusion MRI for Clinical Applications: A Consensus of the ISMRM Perfusion Study Group and the European Consortium for ASL in Dementia”. In: Magnetic resonance in medicine 73.1 (Jan. 2015)
5. F. Heller et al. “Measuring the contrast-enhancement in the skin and subcutaneous fatty tissue with the NMR-MOUSE: a feasibility study”. In: RoFo: Fortschritte Auf Dem Gebiete Der Rontgenstrahlen Und Der Nuklearmedizin 177.10 (Oct. 2005)
Figures