1199
Type-III NUFFT Image Reconstruction for a 65 mT Single-Sided Prostate MRI Scanner with Non-Linear Gradient Fields
Meredith Sadinski1, Ram Narayanan1, Alek Nacev1, and William A Grissom2
1Promaxo, Oakland, CA, United States, 2Biomedical Engineering, Vanderbilt University, Nashville, TN, United States
1Promaxo, Oakland, CA, United States, 2Biomedical Engineering, Vanderbilt University, Nashville, TN, United States
Synopsis
Single-sided low-field MRI scanners can provide image guidance during interventions such as prostate biopsies without restricting surgical access. However, image reconstruction from these scanners is challenging due to the non-linearity of their gradient fields and inhomogeneous B0 fields. At the same time, non-Cartesian sampling is desirable for these scanners to maximize SNR efficiency and provide self-navigation. In this work, we report a fast iterative 3D Type-III NUFFT reconstruction for a 65 mT single-sided prostate imager, with non-linear single-sided gradient fields and a built-in B0 gradient field. The reconstruction was compared with a model-based reconstruction for a high-bandwidth radial RARE acquisition.
Introduction
Single-sided low-field MRI scanners can provide image guidance during interventions such as prostate biopsies without restricting surgical access. However, image reconstruction from these scanners is challenging due to the non-linearity of their gradient fields and inhomogeneous B0 fields. At the same time, non-Cartesian sampling is desirable for these scanners to maximize SNR efficiency and provide self-navigation. In this work, we report an iterative 3D Type-III Non-Uniform Fast Fourier Transform (NUFFT) reconstruction for a low-field single-sided prostate imager, with non-linear single-sided gradient fields and a built-in 58-74 mT B0 gradient field. The reconstruction is compared with an explicit system matrix GPU reconstruction for a radial RARE acquisition.Methods
Imaging Sequence: Figure 2 shows the CHORUS [1] RARE pulse sequence used for data acquisition. The sequence was used to image 6 slabs of an ACR phantom where for each slab it collected 600 total shots across 150 half-spokes with golden angle increments, 4 shots per half-spoke, 7 echoes per shot. It used an echo spacing (ESP) of 5.1 ms and a TR of 1500 ms. The WURST π/2 excitation and π refocusing pulses used n = 40 and 40 kHz bandwidth. Across the six slabs the π/2 pulse durations ranged from 1.7 to 3.0 ms and the π pulse durations ranged from 1.4 to 4.0 ms, which were set based on local B1 field strength. The resulting k-space dataset had dimensions 2 coils x 150 radial half-lines x 4 shots x 7 echoes x 40 readout/frequency encoded points.Type-III NUFFT: Figure 3 shows the steps of the 3D Type-III NUFFT which relates image-space data collected with non-linear gradients (NLG) and a static B0 field/Gz gradient to non-Cartesian k-space data. The algorithm follows that of Ref. [2] (which was originally proposed for NLG-corrected non-Cartesian image reconstruction on a head-only 3T scanner) and was implemented in SigPy [3]. The forward operation takes an input image stack on Cartesian (x,y,z) coordinates and grids it to a 1.5x-oversampled grid using the non-linear gradient fields. Gridding is performed with Kaiser Bessel functions with a kernel width of 4. The gridded image is pre-deapodized for later k-space gridding, the FFT is calculated, and the result is gridded to the non-Cartesian trajectory. The non-Cartesian signal is deapodized for the previous image gridding, resulting in the output k-space data. The NUFFT was implemented as a linear operator in SigPy and the steps were reversed for the transpose operation.
Reconstruction: The Type-III NUFFT was applied in a conjugate gradient algorithm with 3 iterations to reconstruct the k-space data to a Cartesian grid, using gradient and B0/Gz maps measured using a 3-axis Hall probe. The measured gradient fields were projected onto the B0 vector at each location to obtain the gradient fields used for reconstruction. The fields and radial phase encoding coordinates were used to reconstruct images onto a 120 x 120 x 40 array with resolution 1.6 x 1.6 x 2.8 mm3. Images were reconstructed separately for each coil and then combined using ESPIRiT [4], then the 6 stacks of images for each slab were interpolated to a single image volume. Reconstructions were performed on an Intel Core i7-10750H laptop using an NVIDIA RTX2070 GPU with 8GB RAM (HP, Palo Alto, CA, USA) for NUFFT calculations. An iterative reconstruction using explicit system matrix multiplies on the GPU was also performed for comparison.
Results
Averaged over 100 repetitions, the average forward Type-III NUFFT took 32.3 ms on the GPU, while the average transpose Type-III NUFFT took 35.3 ms. In comparison, the explicit system matrix forward multiplies took 3.36 s (100x longer) and the transpose multiplies took 15.4 s (400x longer). Figure 4 shows reconstructed images using both techniques. The NUFFT images have higher contrast between the bright and dark regions of the phantom, and less signal blooming outside. Both images have a mottled noise appearance, which is likely caused by incoherent leaked and un-encoded signals due to a lack of gradient crushing.Discussion
A 3D Type-III NUFFT reconstruction was implemented on a GPU for a 58-74 mT single-sided prostate MRI scanner and validated in reference to an explicit system matrix reconstruction. The NUFFT operations were more than 100x faster than the explicit system matrix multiplications (which were also implemented on the GPU), and produced images with improved contrast. Future work will optimize density compensation and the number of CG iterations, while modeling signal leakage and un-encoded signal in each echo, in order to reduce noise-like artifacts in the images.Acknowledgements
NIH R01 EB 030414References
- M. Foroozandeh, M. Nilsson, and G. A. Morris. Improved ultra-broadband chirp excitation. J Magn Reson, 302:28–33, 2019.
- S. Tao, J. D. Trzasko, T. Shu, J. Huston III, K. M. Johnson, P. T. Weavers, E. M. Gray, and M. A. Bernstein. NonCartesian MR image reconstruction with integrated gradient nonlinearity correction. Med Phys, 42(12):7190–7201, 2015.
- F. Ong and M. Lustig. SigPy: A Python Package for High Performance Iterative Reconstruction. Proc. Intl. Soc. Mag. Reson. Med. 27 (2019), p. 4819.
- M. Uecker, P. Lai, M. J. Murphy, P. Virtue, M. Elad, J. M. Pauly, S. S. Vasanawala, and M. Lustig. ESPIRiT – An eigenvalue approach to autocalibrating parallel MRI: Where SENSE meets GRAPPA. Magn Reson Med, 71(990–1001), 2014.
Figures
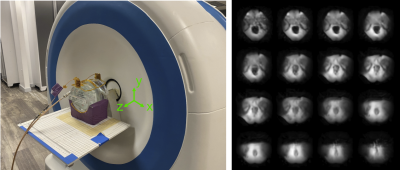
Figure 1: Left: Scanner setup for radial RARE imaging of an ACR phantom. The phantom is imaged with a 2-channel receiver coil array. The patient lies supine with legs up and their prostate in front of the magnet’s opening, which provides surgical access. There is a built-in z-gradient field that is used for frequency encoding in concert with pulsed x- and y-gradient phase encoding. Right: Representative prostate images from the scanner, using a model-based reconstruction.
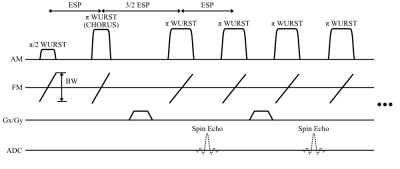
Figure 2: CHORUS RARE interleaved center-out radial pulse sequence. A π/2 WURST excitation pulse is followed by a π WURST CHORUS pulse which compensates its quadratic phase. Phase encoding gradients are applied between every other π WURST pulse pair during the spectral echoes which are not recorded. The spin echo signals between every other pulse are recorded and are both phase-encoded and frequency encoded in the slice dimension by the magnet’s built-in z-gradient.
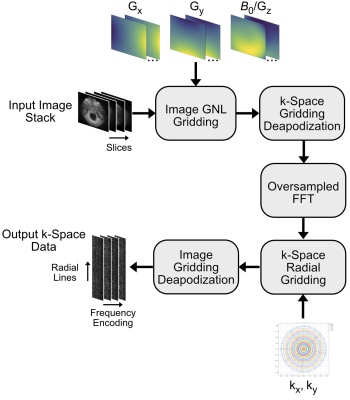
Figure 3: 3D Type-III NUFFT steps. The forward Type-III NUFFT takes an input image stack on Cartesian (x,y,z) coordinates and grids it to an oversampled grid using the non-linear gradient fields. The gridded image is pre-deapodized for the later k-space gridding, the FFT is calculated, and the result is gridded to the non-Cartesian trajectory. The non-Cartesian signal is deapodized for the previous image gridding, resulting in the output k-space data.
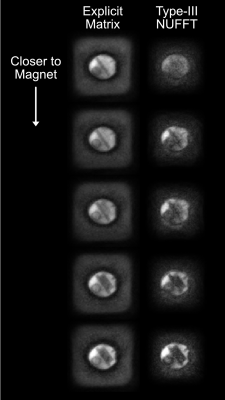
Figure 4: Representative reconstructed images from the final 40-slice volume using an explicit system matrix and the 3D Type-III NUFFT, both with 3 CG iterations. The NUFFT images have improved contrast between the bright and dark regions of the phantom, and reduced signal blooming outside.
DOI: https://doi.org/10.58530/2022/1199