1196
Fast 3D Passive Needle Localization for MR-Guided Interventions using Radial White Marker Acquisitions and CNN Postprocessing
Jonas F. Faust1,2, Axel J. Krafft2, Daniel Polak2, Ralf Vogel3, Peter Speier2, Nicolas G. R. Behl2, Mark E. Ladd4, and Florian Maier2
1Ruprecht-Karls-Universität Heidelberg, Heidelberg, Germany, 2Siemens Healthcare GmbH, Erlangen, Germany, 3Pattern Recognition Lab, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany, 4Medical Physics in Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany
1Ruprecht-Karls-Universität Heidelberg, Heidelberg, Germany, 2Siemens Healthcare GmbH, Erlangen, Germany, 3Pattern Recognition Lab, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany, 4Medical Physics in Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany
Synopsis
Accurate 3D localization of biopsy needles during MR-guided interventions is especially challenging due to time restrictions in real-time workflows. While active tracking methods rely on additional RF-sensitive hardware, rapid passive tracking methods often make use of markers or prior knowledge regarding the needle location. In an ex-vivo study, we investigated a novel passive tracking method using heavily undersampled, radial acquisitions in combination with contrast-optimized White Marker imaging and CNN image postprocessing regarding its potential to speed up passive needle artifact localization without the use of additional hardware or prior knowledge on the needle location.
Introduction
For MR-guided in-bore needle interventions, typically 2D real-time imaging is used for guidance. Due to 2D imaging, the needle will not be visible if the imaging planes are not aligned appropriately. Accurate needle localization can support the interventionalist by enabling automatic alignment of the imaging planes with the needle. Techniques used for needle localization in real-time workflows achieve acquisition times below one second but often require the use of additional markers/hardware or prior knowledge on the needle location.1-3 Recently, the use of Convolutional Neural Networks (CNNs) has been introduced for the automatic segmentation of needle artifacts in 3D MR images (acquisition time: 1min), allowing for the accurate path localization of MR-compatible needles4, and has also been shown to enable 2D needle tracking5,6. We investigated contrast-optimized White Marker7, radial acquisitions combined with CNN-based image postprocessing in an ex-vivo study using porcine phantoms to explore the feasibility of rapid 3D passive needle localization without the use of additional markers or prior knowledge on the needle location.Methods
A prototype sequence was implemented on a 1.5T MAGNETOM Sola system (Siemens Healthcare GmbH, Erlangen, Germany). A White Marker (WM) gradient moment7 was added to the z-gradient in a FLASH sequence before readout (Fig. 1) to compensate susceptibility-induced field inhomogeneities close to the needle, rendering a sparse image contrast with a bright needle artifact on dark, dephased anatomical background (Fig. 2c). Using a 3D golden means radial acquisition8 allows for retrospective undersampling. A 3D U-Net9 was implemented and trained to perform a regression towards the presumed needle artifact location based on the reconstructed images. A principal component analysis (PCA) allowed determination of both the centroid and orientation of the (thresholded) U-Net output.The proposed tracking technique was evaluated in an ex-vivo study. To train the U-Net, an MR-compatible needle (KIM16/14, ITP GmbH, Bochum, Germany) was inserted into six porcine phantoms (Fig. 2a,2b), obtaining 284 different needle poses (90% training set, 10% validation set) with the phantoms rotated by a random angle around the vertical axis for every new needle pose (Fig. 3). An additional phantom was used to evaluate the performance of the localization method (test set with 35 needle poses). Entry point and tip of the needle artifact were manually annotated on images reconstructed from the fully sampled data sets to generate ground truth labels (Fig. 2c,2d). Images were acquired with k-space fully sampled (6434 projections10) and retrospectively undersampled to 500, 200, 100, 50 and 20 projections, corresponding to acquisition times of 76.1s, 6.6s, 3.1s, 2.0s, 1.4s and 1.0s (measurement times including unused WM-encodings in x- and y-direction and 200 dummy pulses). The U-Net was specifically trained for the different undersampling factors, using an L2 loss function and a Stochastic-Gradient-Decent (SGD) optimizer. Performance of the needle artifact localization method was compared using two measures (Fig. 5a), the angle α between the annotated needle artifact and the U-Net prediction and the distance Δx between the predicted needle path and the center point of the manual annotation. The measures were used to assess the feasibility of automatic slice positioning using the proposed technique, as they allow estimation of the required slice thickness Δs to cover the entire needle artifact with length l:
$$\Delta s=\Delta x+\frac{l}{2}\cdot\sin{\alpha}$$
Measurements were performed using a 20-channel head coil. Image matrix size = (64px)3; FOV = (300mm)3; res = (4.7mm)3; flip angle = 5°; WM gradient moment = 5.008mT∙m-1∙ms; TR = 3.9ms; TE = 1.65ms, bandwidth = 900Hz.
Results
Fig. 4 shows the method’s performance for a representative sample from the test set. Fig. 5b and 5c compare Δx and α evaluated over the test set for various undersampling factors. For the fully sampled images, a median accuracy of (3.1±0.7)mm for Δx and a median accuracy of (3.8±2.1)° for α were found (median given with the respective median absolute deviation). For the images reconstructed from a subset of 20 projections, a median accuracy of (4.0±1.2)mm and (7.8±4.5)° was found. For a potential application of the localization method to automatically position a 2D imaging slice, a maximum required slice thickness of 6.4mm and 10.8mm is estimated for 6434 and 20 projections, assuming a needle path length of 10cm.Discussion
We successfully developed a novel method for rapid passive needle localization and evaluated the technique in ex-vivo phantoms. Automatic slice positioning appears feasible with the slice thickness chosen appropriately. A significant speed-up of the needle localization could be achieved using high undersampling factors. Reduction of the acquisition time to less than 1/3 is probably feasible by removing unused acquisitions. A further improvement of the achieved accuracy is expected to be feasible with better label quality, e.g., by annotating the needle location on additionally acquired Fast-Spin-Echo reference scans.Conclusion
The developed technique allows 3D image-based needle artifact localization in ex-vivo phantoms and provides sufficient accuracy for automatic slice positioning. A potential speed-up of the technique can be achieved by heavy undersampling as demonstrated in the retrospective data analysis. Therefore, the concept appears promising for further optimization and integration into real-time workflows. It will be evaluated in-vivo as a next step.Acknowledgements
The authors thank Dr. Heinz-Werner Henke (Innovative Tomography Products GmbH, Bochum, Germany) for providing the MR-compatible needle.References
- de Oliveira A, Rauschenberg J, Beyersdorff D et al. Automatic passive tracking of an endorectal prostate biopsy device using phase-only cross-correlation. Magnetic Resonance in Medicine 2008;59:1043-1050.
- Reichert A, Reiss S, Krafft, AJ et al. Passive needle guide tracking with radial acquisition and phase-only cross-correlation. Magnetic Resonance in Medicine 2021;85(2):1039-1046.
- Patil S, Bieri O, Jhooti P and Scheffler, K. Automatic slice positioning (ASP) for passive real-time tracking of interventional devices using projection-reconstruction imaging with echo-dephasing (PRIDE). Magnetic Resonance in Medicine 2009;62: 935-942.
- Mehrtash A, Ghafoorian M, Pernelle G et al. Automatic Needle Segmentation and Localization in MRI With 3-D Convolutional Neural Networks: Application to MRI-Targeted Prostate Biopsy. IEEE Transactions on Medical Imaging 2019;38(4):1026-1036.
- Weine J, Schneider R, Kägebein U et al. Interleaved White Marker Contrast with bSSFP Real-Time Imaging for Deep Learning based Needle Localization in MR-Guided Percutaneous Interventions. ISMRM 27th Annual Meeting & Exhibition 2019.
- Li X, Young AS, Raman SS et al. Automatic needle tracking using Mask R-CNN for MRI-guided percutaneous interventions. International Journal of Computer Assisted Radiology and Surgery 2020;15:1673-1684.
- Seppenwoolde JH, Viergever MA, Bakker CJ. Passive tracking exploiting local signal conservation: the white marker phenomenon. Magnetic Resonance in Medicine 2003;50(4):784-790.
- Chan RW, Ramsay EA, Cunningham CH et al. Temporal stability of adaptive 3D radial MRI using multidimensional golden means. Magnetic Resonance in Imaging 2009;61(2):354-363.
- Çiçek Ö, Abdulkadir A, Lienkamp S et al. 3D U-Net: Learning Dense Volumetric Segmentation from Sparse Annotation. Medical Image Computing and Computer-Assisted Intervention - MICCAI 2016;424-432.
- Park J, Lee J, Lee J et al. Strategies for rapid reconstruction in 3D MRI with radial data acquisition: 3D fast Fourier transform vs two-step 2D filtered back-projection. Scientific Reports 2020;10:13813.
Figures
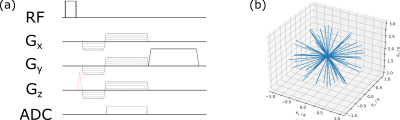
Figure 1: (a)
Pulse diagram of implemented sequence: An additional WM gradient (red) was
combined with radial FLASH acquisitions to refocus part of the magnetization which
was dephased due to inhomogeneities in B0 around the needle. (b) k-space
diagram showing readout trajectory. Spoke distribution allowed retrospective
undersampling by choosing an arbitrary continuously acquired subset of projections. Readout
appears shifted off-center in kz direction due to WM gradient.

Figure
2: (a)
Needle inserted in porcine tissue phantom. (b) Phantom placed in head coil. Needle
pose and phantom’s orientation were changed for each acquired image. (c) Generated
contrast (single transversal slice, fully sampled k-space) with refocused signal
near needle and dephased background signal from tissue for example needle pose.
Artifact position was annotated by marking entry (blue crosshair) and tip point
(red crosshair). (c) Generated label, connecting marked points with a gaussian-blurred
line.

Figure
3: Descriptive
analysis of the training/validation and the test set. (a) Overlay of all manually
annotated needle paths within the chosen FOV. (b) Distribution of needle path
lengths. (c) Angular distribution of the different needle trajectories.
Inclination angle Θ
describes the angle between the needle path and the transversal plane. The
azimuthal angle Φ describes the angle between the projection of the needle path onto
the transversal plane and the y-axis.
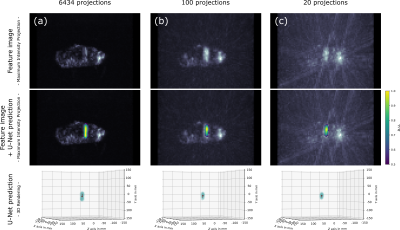
Figure
4: Representative
sample from the test set. The feature image was reconstructed from all 6434 acquired
projections (a), a subset of 100 projections (b) and a subset of 20 projections
(c). Analyzing the corresponding U-Net prediction (normalized & thresholded)
using a PCA, we obtain a distance of 3.7mm, 4.0mm and 4.1mm between the ground truth
needle center point and the predicted needle path (Δx),
and an angular deviation between the ground truth and the predicted needle path (α)
of 2.4°, 2.8° and 3.3° for (a), (b) and (c), respectively (cf. Fig. 5a).
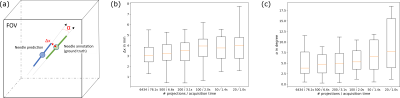
Figure
5: (a)
As measures to evaluate the method's performance for automatic 2D slice positioning, we
determine the angle α between the ground truth needle artifact annotation and
the predicted needle path as well as the distance Δx
between the center point of the annotation and the predicted needle path. Δx (b) and α (c) evaluated over the test set for different undersampling factors. Median depicted as orange line, boxes extend from lower
to upper quartile values of data, whiskers add 1.5x the interquartile range.
DOI: https://doi.org/10.58530/2022/1196