1192
Towards 15N molecular MRI with 15N-nicotinamide hyperpolarized by dissolution dynamic nuclear polarization (dDNP)1SBMI, MOIN CC, UKSH, Kiel University, Kiel, Germany, 2Otto Diels Institute for Organic Chemistry, Kiel University, Kiel, Germany, 3Universität zu Lübeck, Lübeck, Germany, 4Institute of Clinical Molecular Biology, UKSH, Kiel University, Kiel, Germany
Synopsis
We synthesized 1-15N nicotinamide (NA) and built a saddle-shaped mouse body linear coil for 15N imaging. We found a way to polarize 1-15N-NA by dissolution dynamic nuclear polarization to 9.1±4.4% and measured the effect of the radical load. The lifetime of hyperpolarized NA was field-dependent: 100 s at 1 T, 60 s at 7 T, and 30 s at 9.4 T. An effect of the pH on liquid-state polarization was found. N FLASH MRI of hyperpolarized NA was acquired in less than 1 s.
Introduction
The hyperpolarization of nuclear spins boosts the MR signal of selected molecules and has enabled real-time metabolic imaging in vivo.[1,2] 1-13C-pyruvate has the most striking applications in cancer diagnosis by measuring the activity of lactate dehydrogenase.[2] Most often, 13C was hyperpolarized, with some examples for 1H, 15N, and other nuclei.[3,4] 15N is an attractive alternative for molecules that do not have long-lived 13C or where the label or polarization does not survive a chemical transformation.Hyperpolarized nicotinamide (NA) is a promising imaging target as it is a critical molecule in the glycolysis and metabolism of fatty acids. NA can be used to spy on the activity of several enzymes: nicotinamide N-methyltransferase[5], nicotinamide phosphoribosyltransferase[6], nicotinamide amidohydrolase, and cytochrome P450.
We present the hyperpolarization of 1-15N-NA for the first time, using dynamic nuclear polarization (dDNP), and demonstrate in vitro 15N-MRI.
Methods
NMR and MRI: 15N signals were acquired using a 1T 15N benchtop NMR (Spinsolve), 7T MRI (BioSpec 70/30), and 9.4T WB NMR (Avance NEO), the manufacturer’s software, and MestReNova.dDNP. All dDNP experiments were performed a cryogen-free dDNP system (SpinAligner)[10] at ~1.4K and 6.7T with a microwave frequency of 187.185GHz at 35.2W and ~50mg samples. The built-up of the 15N polarization in the solid-state was monitored every two minutes with a ~3.5° pulse. After dissolution with 5mL superheated dissolution media (Neutral-DM: pH 5.13, Basic-DM: pH 12.6), the sample was transferred to the detection site in ~30s.
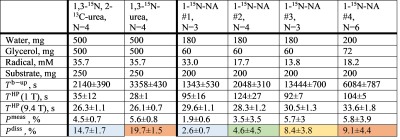
15N-contrast agents (CA). 1-15N-NA was synthesized from NA via spontaneous exchange with 15NH4 in methanol,[9] resulting in a white powder with a yellow tint at 40% yield and 91±2 % 15N enrichment. We mixed 1,3-15N-urea (Sigma-Aldrich, CAS: 2067-80-3), 1,3-15N, 2-13C-urea (Sigma-Aldrich, CAS: 58069-83-3) or 1-15N-NA with trityl radical (AH111501, POLARIZE) in deionized water and glycerol (Figure 1).
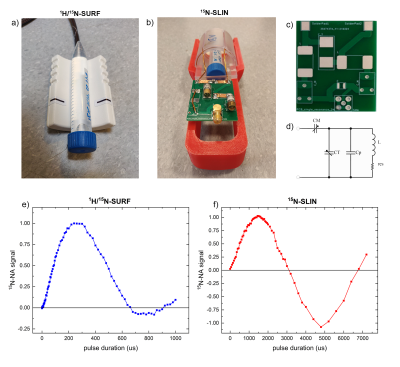
15N MRI probes. 1H-15N, fixed dual-tune linear surface coil (1H/15N-SURF, 30 mm, O-XL-HL-070, Rapid Biomedical, Figure 3a) was compared to an in-house-built 3D-printed, linear 15N saddle-shaped coil (15N-SLIN, L=52 mm, ID=46 mm, Figure 3b) in cross-coil mode with a 1H quadrature resonator (112/086 QSN, Bruker). 15ml aqueous solution containing 800mM 15NH4 with 3 vol% Gd-contrast ([Gd], 1mmol/ml, Gadovist, Bayer) was used as a model solution.
Results
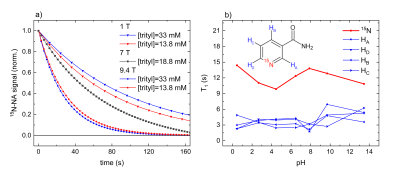
(13C)-15N-Urea polarization. 15N2-urea and 13C-15N2-urea were reliably polarized to P15N = 3.8 - 6.4 % (N=8, measured 30 s after dissolution), with higher polarization (4.5±0.7 % vs 5.6±0.8%) and longer dDNP build-up constants Tb-up = (2140±390s vs 3358±430s) for 12C isotopomer (Figure 1). The relaxation times at 1T and 9.4T were similar and about 30s; with this lifetime, the back-calculated polarization at the time of dissolution was close to 20%.15N-NA polarization. We carried out three to six dDNP experiments using four different 15N-NA compositions with basic-DM (Figure 1); no liquid-state polarization was observed when neutral-DM was used. The pH for neutral-DM and basic-DM after the dissolution was 7.1 and 12.4, respectively. Reducing the trityl radical concentration from 33mM to 13.8mM increased the 15N polarizations Pdiss from 2.6% to 8.4%, build-up constants Tb-up from 0.4h to 3.7h. T1 was strongly field-dependent: 100s at 1T, 60s at 7T, and 30s at 9.4T (Figure 2a). We could not observe a change in T1 and polarization levels when deuterated dissolution media or a different radical amount was used. To investigate the influence of pH, we measured T1 of a 200mM 1-15N-NA sample (in H2O:D2O = 9:1) in thermal equilibrium at 9.4T as a function of pH (Figure 2b); no significant T1 variation was found.
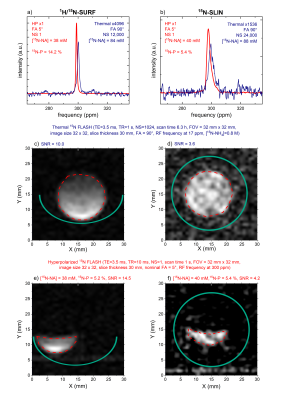
15N MRI and probes. 1H/15N-SURF provided 2.8 times higher, but much less homogeneous signals than 15N-SLIN (Figure 3, spectroscopy of 1 cm slice parallel to coil). Both allowed 15N-spectroscopy (Figure 4a,b) and 15N-MRI (FLASH[11]) using the 15NH4 model solution in thermal equilibrium (Figure 4c,d). Subsecond 15N-MRI of hyperpolarized 15N-NA provided very high SNR (Figure 4e,f).
Discussion
15N probes. The in-house 15N-SLIN coil can be built in one day and at the cost of only about 500 Euro (mainly capacitors); it features about 3 times lower SNR and longer FA for the same power as a commercial probe (Figure 3,4). Efforts are currently undertaken to improve SNR and reduce FA.Hyperpolarization of 15N-NA. 15N-NA was successfully synthesized and polarized for the first time. Already now, the polarization of ~10% is approaching the levels needed for in vivo MRI; high SNR MRI was achieved in vitro in less than a second. We assume that the reduced built-up time and polarization of the dual-label urea were caused by an increased solid-state relaxation. Variation of the sample composition is expected to increase the polarization yield further (water/glycerol ratio, DMSO). Previously, 3.4% 15N-NA polarization was achieved in methanol using parahydrogen-induced polarization.[9] The protocols presented here are much closer to an in vivo application, although the pH neutralizing step was not yet tested.
Acknowledgements
We acknowledge support by funding from the German Federal Ministry of Education and Research (BMBF) within the framework of the e:Med research and funding concept (01ZX1915C), the Emmy Noether Program "metabolic and molecular MR" (HO 4604/2-2), the research training group "materials for brain" (GRK 2154/1-2019), the DFG grant INST 257/616-1 (FUGG), the SFB "bulk-reaction" (TRR 287), the clusters of excellence "precision medicine in inflammation" (PMI 1267) and "inflammation at interfaces" (EXC 306, FOR 5042). Kiel University and the Medical Faculty are acknowledged for supporting the Molecular Imaging North Competence Center (MOIN CC) as a core facility for imaging in vivo. MOIN CC was founded by a grant from the European Regional Development Fund (ERDF) and the Zukunftsprogramm Wirtschaft of Schleswig-Holstein (Project no. 122-09-053).References
[1] P. Bhattacharya, K. Harris, A. P. Lin, M. Mansson, V. A. Norton, W. H. Perman, D. P. Weitekamp, B. D. Ross, MAGMA 2005, 18, 245–256.
[2] S. J. Nelson, J. Kurhanewicz, D. B. Vigneron, P. E. Z. Larson, A. L. Harzstark, M. Ferrone, M. van Criekinge, J. W. Chang, R. Bok, I. Park, G. Reed, L. Carvajal, E. J. Small, P. Munster, V. K. Weinberg, J. H. Ardenkjaer-Larsen, A. P. Chen, R. E. Hurd, L.-I. Odegardstuen, F. J. Robb, J. Tropp, J. A. Murray, Sci. Transl. Med. 2013, 5, 198ra108-198ra108.
[3] J. Milani, B. Vuichoud, A. Bornet, R. Melzi, S. Jannin, G. Bodenhausen, Rev. Sci. Inst. 2017, 88, 015109.
[4] E. H. Suh, J. M. Park, L. Lumata, A. D. Sherry, Z. Kovacs, Chem. Commun. 2020, 3, 1–9.
[5] S. Aksoy, C. L. Szumlanski, R. M. Weinshilboum, J. Biol. Chem. 1994, 269, 14835–14840.
[6] I. Shin-ichiro, Curr. Pharm. Des. 2008, 15, 20–28.
[7] K. Yaku, K. Okabe, K. Hikosaka, T. Nakagawa, Front. Oncol. 2018, 8, 622.
[8] A. A. Pramono, G. M. Rather, H. Herman, K. Lestari, J. R. Bertino, Biomolecules 2020, 10, 358.
[9] R. V. Shchepin, D. A. Barskiy, D. M. Mikhaylov, E. Y. Chekmenev, Bioconjugate Chem. 2016, 27, 878–882.
[10] J. H. Ardenkjær‐Larsen, S. Bowen, J. R. Petersen, O. Rybalko, M. S. Vinding, M. Ullisch, N. C. Nielsen, Magn. Reson. Med. 2019, 81, 2184–2194.
[11] J. Frahm, A. Haase, D. Matthaei, Magn. Reson. Med. 1986, 3, 321–327.
Figures