1191
15N-enriched NAD+/ NADH Analogs as Redox-Responsive MRI Sensors1Advanced Imaging Research Center, UT Southwestern Medical Center, Dallas, TX, United States, 2Chemistry and Biochemistry, The University of Texas at Dallas, Richardson, TX, United States, 3Physical Measurement Laboratory, National Institute of Standards and Technology, Boulder, CO, United States, 4Phillips Medical Systems, Cleveland, OH, United States
Synopsis
In this study, we prepared a 15N-enriched analog of NAD+ (N-methyl nicotinamide = MNA+) and demonstrated that it undergoes reduction by sodium dithionate to form MNAH. The 15N chemical shifts of the oxidized and reduced forms differ by 124.2ppm. DNP of MNA+ followed by dissolution and 15N NMR showed a favorable T1 relaxation time of 130s at 1T and 50s at 3T. Deuteration of the methyl protons only increased the T1 of 15N by ~10s. The long T1 of 15N in these NAD+/NADH mimetics and the large chemical shift difference offer the exciting potential for their use as redox sensors.
Introduction
Redox responsive sensors for imaging the tissue redox state are highly desirable for the detection and assessment of numerous clinical conditions including hypoxia in cancer, oxidative stress in fatty liver disease, and neurodegenerative diseases such as Alzheimer’s1-4. The ability to noninvasively image oxidative stress would provide clinicians with a powerful tool for monitoring disease progression and track patient responsiveness throughout the course of treatment. One approach to assess oxidative stress is by measuring the NAD+/NADH ratio5,6. NAD+ and NADH are key enzymatic cofactors responsible for the maintenance of the redox state of the cell as well as for the regulation of cellular metabolism and processes. As cells undergo oxidative stress, the reduced form, NADH, is depleted resulting in an imbalanced NAD+/NADH ratio7. The goal is to synthesize a probe which would reflect this dynamic redox chemistry and allow us to measure the cellular NAD+/NADH in vivo. Several groups have previously demonstrated the NAD+/NADH mimetics, MNA+/MNAH, are readily synthesized and can be used as redox indicators8-12. Here we report our results on the synthesis of 15N labeled MNA derivatives as well as conventional and hyperpolarized 15N NMR studies. Hyperpolarized NMR technology (microwave driven dynamic nuclear polarization) has been used to amplify NMR signal detection of 15N dramatically thereby allowing potential imaging applications of this low sensitivity nucleus13-16. As the hyperpolarized magnetization decays by T1 (spin-lattice) relaxation, the use of 15N in hyperpolarized NMR/MRI is promising because of the long T1 of many 15N compounds13,17,18. In addition, 15N compounds also exhibit larger chemical shift differences compared to 13C and have little to no background signal from natural abundance 15N. Thus, 15N-enriched NAD+/NADH mimetics have potential to serve as redox active sensors for hyperpolarized 15N MRS/MRI studies.Methods
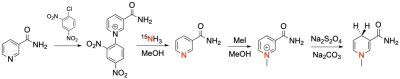
The oxidized NAD+ mimetic, [1-15N]-3-carbamoyl-1-methylpyridin-1-ium-iodide (MNA+), was synthesized from 15N labeled nicotinamide via N- quaternization using iodomethane according to previously described methods19. MNA+ was reduced with sodium dithionite to afford the reduced form, [1-15N]-3-carbamoyl-1,4-dihydro-1-methylpyridine (MNAH). MNA+ and MNAH were characterized by 1H, 13C, and 15N NMR and mass spectrometry. The oxidized form was polarized by dynamic nuclear polarization (DNP) in a clinical polarizer (SPINlab) using trityl OX063 free radical as the polarizing agent followed by dissolution with superheated water. After dissolution, the hyperpolarized 15N signal was recorded at 1 T using a benchtop 15N Magritek NMR spectrometer. In an attempt to further prolong the T1 of the 15N probe, the deuterated form of the compound [1-15N]-3-carbamoyl-1-(methyl-d3)pyridin-1-ium iodide was synthesized using d3-iodomethane. The hyperpolarized 15N signal was recorded at 1 T after dissolution using a benchtop 15N Magritek NMR spectrometer and at 3 T using a Philips clinical scanner fashioned with a custom built 15N coil.Results and Discussion
The aim of this project was to develop 15N NMR probes that mimic the redox action of the cofactors, NAD+/NADH. Conventional 15N NMR spectroscopy at 9.4 T showed a single resonance for MNA+ at 180.7 ppm while the reduced product, MNAH showed a single resonance at 56.5 ppm. This dramatic chemical shift difference of 124.2 ppm makes this redox pair highly desirable for in vivo chemical shift imaging. The decay of the hyperpolarized 15N signal of MNA+ afforded a spin-lattice (T1) relaxation time of 130 s at 1 T, a very favorable T1 for HP imaging. Deuteration of the methyl group increased the 15N T1 to only 140 s, indicating that chemical shift anisotropy (CSA) is the main relaxation mechanism for the 15N label in this molecule. This was confirmed by measuring the T1 of the deuterated form using a clinical 3 T scanner. The measured value at this field was 50 s. Current work is focused on studying this redox chemistry under different reduction conditions as well as using MNA+/MNAH as cofactors in enzymatic oxidation and reduction reactions.Conclusion
In summary we have synthesized a 15N-enriched mimetic of NAD+ for potential use as a redox active HP probe for 15N MRS/MRI. This probe exhibits a large chemical shift difference of 124.2 ppm when it undergoes reduction and has a long T1 relaxation time of 140 s at 1 T. The long T1 relaxation time and large chemical shift difference between the oxidized and reduced forms demonstrate the exciting potential for their use as 15N NMR/MRI redox sensors.Acknowledgements
This work was supported by a grant from the NIH (P41-EB015908) and the UT-Dallas/UT-Southwestern Green Fellows Program.References
1. Eales KL, Hollinshead KER, Tennant DA. Hypoxia and metabolic adaptation of cancer cells. Oncogenesis. 2016;5(1):e190-e190.
2. iguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, Gargiulo G, Testa G, Cacciatore F, Bonaduce D, Abete P. Oxidative stress, aging, and diseases. Clin Interv Aging. 2018;13:757-772.
3. Chandrasekaran A, Idelchik MDPS, Melendez JA. Redox control of senescence and age-related disease. Redox Biol. 2017;11:91-102.
4. Venkataraman K, Khurana S, Tai T. Oxidative Stress in Aging-Matters of the Heart and Mind. Int J Mol Sci. 2013;14(9):17897-17925.
5. Katsyuba E, Auwerx J. NAD+ Modulation. In Introductory Review on Sirtuins in Biology, Aging, and Disease.Chapter 3, Academic Press ISBN 9780128134993; 2018:27-44.
6. Massudi H, Grant R, Guillemin GJ, Braidy N. NAD+ metabolism and oxidative stress: the golden nucleotide on a crown of thorns. Redox Report. 2012;17(1):28-46.
7. Xie N, Zhang L, Gao W, et al. NAD+ metabolism: pathophysiologic mechanisms and therapeutic potential. Sig Transduct Target Ther. 2020;5(1):227
8. Paul CE, Churakova E, Maurits E, Girhard M, Urlacher VB, Hollmann F. In situ formation of H2O2 for P450 peroxygenases. Bioorg Med Chem. 2014;22(20):5692-5696.
9. Ryan JD, Fish RH, Clark DS. Engineering Cytochrome P450 Enzymes for Improved Activity towards Biomimetic 1,4-NADH Cofactors. ChemBioChem.2008;9(16):2579-2582.
10. Nowak C, Beer B, Pick A, Roth T, Lommes P, Sieber V. A water-forming NADH oxidase from Lactobacillus pentosus suitable for the regeneration of synthetic biomimetic cofactors. Front Microbiol. 2015;6:957
11. Paul CE, Isabel W. C. E, Arends, Hollmann F. Is Simpler Better? Synthetic Nicotinamide Cofactor Analogues for Redox Chemistry. ACS Catalysis. 2014;4(3):788-797.
12. Knaus T, Paul CE, Levy CW,Vries SD, Mutti FG, Hollmann F, Scrutton NS. Better than Nature: Nicotinamide Biomimetics That Outperform Natural Coenzymes. J Am Chem Soc. 2016;138(3):1033-1039.
13. Jiang W, Lumata L, Chen W, Zhang S, Kovacs Z, Sherry AD, Khemtong C. Hyperpolarized 15N-pyridine Derivatives as pH-Sensitive MRI Agents. Scientific Reports. 2015;5(1):9104.
14. Ardenkjae-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, Servin R, Thaning M, Golman K Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc Nat Aca Sci. 2003;100(18):10158-10163.
15. Day SE, Kettunen MI, Gallagher FA, Hu DE, Lerche M, Wolber J, Golman K, Ardenkjaer-Larsen JH, Brindle KM. Detecting tumor response to treatment using hyperpolarized 13C magnetic resonance imaging and spectroscopy. Nat Med. 2007;13(11):1382-1387.
16. Comment A, Merritt ME. Hyperpolarized Magnetic Resonance as a Sensitive Detector of Metabolic Function. Biochemistry. 2014;53(47):7333-7357.
17. Shchepin RV, Barskiy DA, Mikhaylov DM, Chekmenev EY. Efficient synthesis of nicotinamide-1-15N for ultrafast NMR hyperpolarization using parahydrogen. Bioconjug Chem. 2016;27(4):878-882.
18. Gabellieri C, Reynolds S, Lavie A, Payne GS, Leach MO, Eykyn TR. Therapeutic target metabolism observed using hyperpolarized 15N Choline. J Am Chem Soc. 2008;130(14):4598-4599.
19. Oppenheimer NJ, Matsunaga TO, Kam BL. Synthesis of 15N-1 nicotinamide. A general, one step synthesis of 15N labeled pyridine heterocycles. J Label Compound and Radiopharm. 1978;15(S1):191-196.
Figures
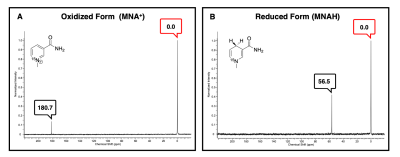
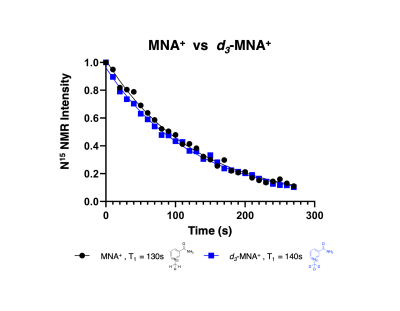
Effect of Deuteration on T1 decay time. Spectra were collected at 1 T every 10 seconds using a 5 degree flip angle.
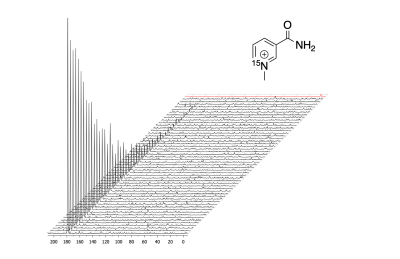
Decay of hyperpolarized magnetization of MNA+ at 1 T. Spectra were collected very 5 seconds using a 5 degree flip angle and referenced to 2.0 M 15NH4Cl (0.0 ppm) reference shown in red.