1190
Simultaneous fMRI and metabolic MRS of hyperpolarized [1-13C]pyruvate during nicotine stimulus in rat1A. I. Virtanen Institute, University of Eastern Finland, Kuopio, Finland
Synopsis
fMRI is widely used to study cerebral metabolism to stimulus through combination of BOLD and hemodynamic changes. Hyperpolarized carbon can also be used to study cerebral metabolism, but it is currently poorly understood. Here we performed simultaneous 1H fMRI and 13C MRS experiments to compare their response to nicotine stimulus. Cortical BOLD signal increase was accompanied by increased bicarbonate labelling. Combined 1H fMRI and 13C MRS may therefore offer complementary information on brain response to stimulus.
Introduction
Nicotine is often used as a stimulant in fMRI as it increases local cerebral glucose utilization on its binding sites1. It induces strong BOLD effect at cortical regions of the brain under urethane anesthesia and also increases CBF and CBV, giving strongest response 1-2 minutes after injection2. BOLD effect is also dose dependent3. Hyperpolarized carbon, so far mainly [1-13C]pyruvate, can be also used to study real-time cerebral metabolism. However, pre-clinically used anesthesia has also an effect on the apparent brain metabolism of hyperpolarized [1-13C]pyruvate possibly via cerebral circulation4,5. In the current study, we performed simultaneous 1H fMRI and metabolic 13C MRS during nicotine stimulus to compare their response.Methods
Six adult female Sprague-Dawley rats (284-346 g) were urethane (ip 1250 mg/kg) anesthetized and their femoral vein and artery were catheterized. Temperature, breath rates and blood gases of animals were monitored during the experiments. [1-13C]pyruvic acid was hyperpolarized with radical AH11501 at 1.35 K, 6.7 T and 188 GHz for 1.5 h in experimental HYPERMAG hyperpolarizer (DTU, Denmark). The sample was dissolved with 0.2 M Tris buffer, neutralized with 2 M NaOH and injected (0.8 mmol/kg) to an animal inside the magnet. During experiment, each animal received two injections of pyruvate (with about 3h apart) preceded by either nicotine (0.25 mg/kg) or saline control injection 60 seconds before hyperpolarized [1-13C]pyruvate injection. The order of nicotine and saline injections was varied between animals. MRI data were collected at 9.4 T using a 1H/13C transmit/receive surface coil and Agilent console. Pulse sequence collected fMRI (GE-EPI, nominal flip angle 60 degrees, TE 20 ms, ten 1.5 mm slices, FOV 32x16mm, matrix 80x40) data followed by one slice-selective (slice 14 mm covering the same region as fMRI slices) 13C MRS spectra (TE=670 µs, nominal flip angle 25 degrees) with one cycle taking 2 s. Fifteen minutes of data were collected with the stimulus injection performed after five minutes and pyruvate injection after six minutes. Regions of interest covering cortical, subcortical and muscle regions were analyzed for fMRI. For 13C, the first 90 seconds following pyruvate injection were summed and peak integrals were calculated.Results
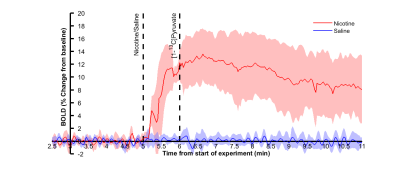
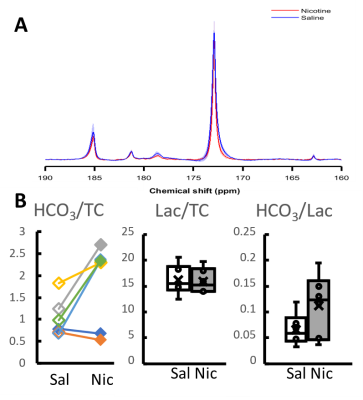
In pilot experiments, simultaneous fMRI and 13C MRS did not lead to degradation of signal compared to individual experiments. Proton signal from cortex increased 12% ± 4% (p<0.01) (Figure 1) following nicotine injection, whereas no subcortical nor intramuscular signal change was observed. Saline injection did not change the observed signal. No change in lactate labelling was observed between saline and nicotine experiments, but an increase in bicarbonate-to-total-carbon ratio (p~0.03) and a trend towards increased bicarbonate-to-lactate (p~0.06) ratios were seen in four out of six rats following nicotine injection (Figure 2).Discussion
Simultaneous fMRI and 13C MRS experiments were feasible in preclinical setting. While a clear cortical fMRI response was observed, MRS responses were more modest. An increase in bicarbonate levels was observed but there was no change in lactate labelling. Unlike in fMRI, only a very narrow time window can be observed with 13C MRS. In the current study, timing between nicotine and pyruvate injections was chosen based on biggest changes on previously reported BOLD responses2, which were similar to the ones seen in the current study. However, brain activation following nicotine injection can change individually and local CBV peaks have been reported even over 10 minutes after nicotine injection6. It is therefore possible that the timing of experiment was not optimal and some other drug or model would lead to stronger responses on metabolic side as well. Alternatively, hyperpolarized glucose might reveal metabolic response more clearly7.Conclusion
1H fMRI and metabolic 13C MRS can be performed simultaneously. This complementary information may allow a better assessment of the brain response to stimulus.Acknowledgements
This research was funded by Academy of Finland (grant #332006).References
1. London ED, Connolly RJ, Szikszay M, Wamsley JK, Damlsb M. Effects of Nicotine on Local Cerebral Glucose Utilization in the Rat. J Neurosci. 1988;8(10):3920-3928.
2. Paasonen J, Salo RA, Shatillo A, et al. Comparison of seven different anesthesia protocols for nicotine pharmacologic magnetic resonance imaging in rat. Eur Neuropsychopharmacol. 2016;26(3):518-531.
3. Bruijnzeel AW, Alexander JC, Perez PD, et al. Acute nicotine administration increases BOLD fMRI signal in brain regions involved in reward signaling and compulsive drug intake in rats. Int J Neuropsychopharmacol. 2014;18(2):1-13.
4. Josan S, Hurd R, Billingsley K, et al. Effects of isoflurane anesthesia on hyperpolarized (13)C metabolic measurements in rat brain. Magn Reson Med. 2013;70(4):1117-1124.
5. Hyppönen V, Stenroos P, Nivajärvi R, et al. Metabolism of hyperpolarised [1– 13 C]pyruvate in awake and anaesthetised rat brains. NMR Biomed. October 2021.
6. Choi JK, Mandeville JB, Iris Chen Y, Kim YR, Jenkins BG. High resolution spatial mapping of nicotine action using pharmacologic magnetic resonance imaging. Synapse. 2006;60(2):152-157.
7. Mishkovsky M, Gusyatiner O, Lanz B, et al. Hyperpolarized 13C-glucose magnetic resonance highlights reduced aerobic glycolysis in vivo in infiltrative glioblastoma. Sci Reports 2021 111. 2021;11(1):1-9
Figures