1188
Hyperpolarized 13C imaging at moderate field strengths: SNR considerations and in-vivo feasibility at 1.5T1Institute for Biomedical Engineering, University and ETH Zurich, Zürich ETH-Zentrum, Switzerland
Synopsis
Motivated by the fact that the hyperpolarized MRI signal does not quadratically scale with field strength as for conventional MRI at thermal equilibrium, the achievable SNR at reduced field strengths is explored. It is demonstrated that for larger coil diameters, sample noise dominates electrical noise at 1.5T and achievable SNR becomes comparable to 3T. By adaptively reducing acquisition bandwidth to the reduced spectral span and given longer T2* decay, effective SNR at 1.5T exceeds 3T. Feasibility is demonstrated with the first in-vivo data acquired on a clinical 1.5T system using hyperpolarized 13C pyruvate.
Introduction
Hyperpolarized 13C in-vivo MRI is usually performed at 3T for clinical and preclinical studies in large animals. Commercial availability of hardware suitable for respective subject sizes with the capability of detecting non-1H nuclei certainly is a limiting factor in this regard. However, as reiterated herein, SNR considerations do favor lower field strengths for larger subjects in case of hyperpolarized imaging.Ongoing clinical trials, especially in cardiac patient cohorts, have meanwhile revealed that B0 inhomogeneities, unreliable electrocardiogram (ECG) triggering and general integration issues into clinical workflow are hampering the translation of hyperpolarized imaging exams at 3T.
This work therefore explores the clinically established field strength of 1.5T (and below) for hyperpolarized 13C imaging based on theoretical considerations and simulations. Furthermore, hyperpolarized 13C in-vivo data acquired on a clinical 1.5T system in a pig model is presented, to the best knowledge of the authors, the first of its kind.
Theory
Intrinsic SNR for hyperpolarized MRI can be described as a function of Larmor frequency $$$\omega$$$, transverse magnetization $$$M_{xy}$$$, coil sensitivity $$$C$$$, as well as coil and sample temperatures $$$T_i$$$, resistances $$$R_i$$$ and the acquisition bandwidth $$$BW$$$:$$SNR(\vec{x})\propto\frac{\omega\cdot\,M_{xy}(\vec{x})\cdot\,C(\vec{x})}{\sqrt{4k_{b}(T_{coil}R_{coil} + T_{sample}R_{sample}) \cdot BW}} $$In contrast to conventional MRI, hyperpolarized $$$M_{xy}$$$ is independent of $$$\omega$$$, as polarization is generated inside the polarizer. Hence the signal scales linearly, not quadratically, with $$$\omega$$$.
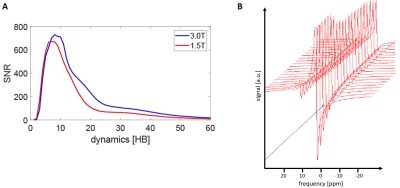
Depending on the coil radius $$$r$$$, wire diameter $$$p$$$, resistivity $$$\rho$$$ and loading with a sample of conductivity $$$\sigma$$$ at distance $$$d$$$ from a single loop coil, two field-dependent noise regimes can be considered for the SNR scaling, in which $$$R_{coil}$$$ or $$$R_{sample}$$$ dominate, as illustrated in Fig-1A:$$R_{coil}=\frac{r}{p}\sqrt{\mu_{0}\omega\frac{\rho}{2}}$$ $$R_{sample}=\frac{2}{3\,pi}\sigma{\omega}{\mu_{0}}^2\omega^2\,r^3\,tan^{-1\frac{\pi\,r}{8d}}$$For small coil diameters, $$${R_{c}\gg\,R_{s}}$$$, leading to an SNR that increases with field strength:$$SNR_{R_{c}\gg\,R_{s}}\propto\frac{\omega}{\sqrt{\sqrt{\omega}\cdot\,BW}}=\frac{\omega^{\frac{3}{4}}}{BW}\overset{adaptive}{\underset{BW scaling}\rightarrow}\,SNR\,\propto\,B_{0}^{\frac{1}{4}}$$whereas in the $$${R_{s}\gg\,R_{c}}$$$ regime, SNR becomes field-independent and solely dependent on bandwidth:$$SNR_{R_{s}\gg\,R_{c}}\propto\frac{\omega}{\sqrt{\omega^2\cdot\,BW}}=\frac{1}{BW}\overset{adaptive}{\underset{BW scaling}\rightarrow}SNR\,\propto\,B_{0}^{-\frac{1}{2}}$$By adaptively changing the acquisition bandwidth inversely proportional to B0, motivated by reduced spectral span and longer $$$T_{2}^{\star}$$$, the effective SNR scaling favors the noise dominated regime at lower frequencies (see Fig-1B).
The clinically relevant range of coil diameters and field strengths between 0.5T and 3T are investigated with respect to their theoretical noise behaviour and relative SNR scaling. For comparison, noise figures of (pre-)amplifiers and other receive chain components are considered constant.
Methods
Intrinsic SNR and noise resistance values were simulated in MATLAB (Mathworks) according to Equations 1-5. A single-looped copper coil with 1mm wire diameter was simulated assuming a semi-infinite sample of muscle1,2. Coil diameters of 5cm, 10cm, 20cm were simulated to reflect clinical and pre-clinical setups with a signal point source positioned 5-10cm from the coil. Synthetic 13C spectra were simulated with fixed and adaptive acquisition BW based on the intrinsic SNR calculations assuming a field-dependent scaling of $$$T2^{\star}=63\cdot\,B_{0}^{-0.964}$$$ for [1-13C]-pyruvate.For in-vivo experiments, [1-13C]-pyruvic acid doped with 15mM trityl radical was polarized in a commercial SpinLab hyperpolarizer (GE Healthcare) and 0.7ml/kg of 250mM neutralized [1-13C]-pyruvate solution were injected 20s post-dissolution into the femoral vein. In-vivo experiments were conducted according to institutional and ethical guidelines.
Imaging and spectroscopy was performed on clinical 1.5T and 3T systems (Philips Healthcare), with a 6-channel Rx coil (r=5cm per element) (Rapid Biomedical) and a 2-channel TxR coil (r=10cm per loop) (PulseTeq), respectively. Dynamic metabolic images were obtained with an echo-shifted EPI sequence, followed by a regularized conjugate gradient reconstruction as described in 3.
Results
Theoretical SNR performance is illustrated in Fig-1. For coil radii ≥5cm, the intrinsic SNR penalty of 1.5T vs. 3T is ≤20%, despite the fact that sample noise is of similar order than coil noise.Fig-2 demonstrates that for certain sequence types such as spectroscopy, adaptively changing acquisition BW can lead to an effective SNR increase of approx. at 1.5T when compared to 3T.
Fig-3 compares flip-angle corrected spectral SNR from data acquired in pigs at 3T and 1.5T. As predicted from simulations, comparable SNR is achieved at identical bandwidths.
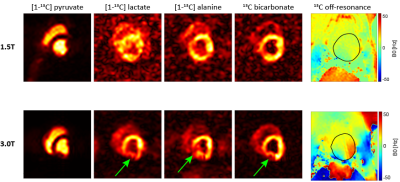
Fig-4 presents the first cardiac in-vivo data acquired at 1.5T alongside 3T data. Image quality and temporal fidelity is consistent with other work performed at 3T. Typical off-resonance effects are noticeably reduced at 1.5T.
Discussion
Simulated SNR and spectra are subject to uncertainty regarding exact field-dependent scaling of relaxation parameters such as T2*. This is however not believed to substantially affect the conclusions drawn herein.Spectral separation by excitation or echo shifting at lower field strengths results in prolonged TE, which in practice limits the available SNR gain. Conversely, as SAR scales quadratically with B0, exploiting higher B1+ can shorten TE. The effective impact on SNR is subject to careful sequence design on a per-case basis.
Receive chain electronics were considered as field-independent with for better comparison. As clinical 1.5T systems are typicallynot optimized for frequencies around 16MHz, there is potential room for improvement.
The current comparison of in-vivo SNR at different field strengths is hampered by the difference in coil setup. Upcoming experiments will focus on thorough comparisons with identical coil geometries.
Conclusion
Hyperpolarized 13C imaging on clinical 1.5T setups is not only feasible but capable of exceeding SNR performance compared to 3T, as well as allows reducing field-dependent artifacts such as B0 inhomogeneities. Thereby current SNR challenges seen in hyperpolarized metabolic imaging trials performed at 3T might be addressed.Acknowledgements
The authors would like to acknowledge Miriam Weisskopf for animal handling and care.References
1. Darrasse L, Ginefri JC. Perspectives with cryogenic RF probes in biomedical MRI. Biochimie 2003;85:915–937. doi: 10.1016/j.biochi.2003.09.016.
2. Hasgall PA, Di Gennaro F, Baumgartner C, Neufeld E, Lloyd B, Gosselin MC, Payne D, Klingenböck A, Kuster N, “IT’IS Database for thermal and electromagnetic parameters of biological tissues,” Version 4.0, May 15, 2018, DOI: 10.13099/VIP21000-04-0. itis.swiss/database
3. Traechtler J, Vishnevskiy V, Fuetterer M, Kozerke S. Joint image and field map estimation for multi‐echo hyperpolarized 13 C metabolic imaging of the heart. Magn. Reson. Med. 2021;86:258–276. doi: 10.1002/mrm.28710.
Figures