1186
Hyperpolarization of 129Xe gas via dissolution DNP: beneficial tips and tricks1LIFMET, École polytechnique fédérale de Lausanne, Lausanne, Switzerland, 2Geneva School of Health Sciences (HES-SO), University of Applied Sciences and Arts Western Switzerland, Geneva, Switzerland
Synopsis
Dissolution DNP (dDNP) is an alternative for hyperpolarizing 129Xe to the well acknowledged method spin exchange optical pumping (SEOP). As opposed to SEOP, DNP takes place in the solid state at very low temperatures. Therefore, it can potentially produce a large volume of hyperpolarized gas after dissolution. In this study, by implementing a new way of preparing the sample, we ease the overall process and establish a superior incorporation of the gas atoms into the solvent. Additionally, due to a surprisingly short radical electron T1, we show that a higher polarization was achieved by applying microwave frequency modulation.
Introduction
Hyperpolarized 129Xe MRI is usually made possible by the well-established spin exchange optical pumping (SEOP) method. At optimal conditions and using 129Xe enriched gas this method can provide up to 3.6L/h of xenon hyperpolarized to 30% 1. An alternative method for hyperpolarizing Xe is dissolution dynamic nuclear polarization (dDNP) followed by a sublimation process. As opposed to SEOP, DNP takes place in the solid state at very low temperature. Therefore, it can potentially produce a large volume of hyperpolarized 129Xe taking advantage from the solid-to-gas transition. Polarization levels of 5-7% were achieved with the promising advantage of using a generic dissolution DNP polarizer 2.There is a maximum Xe concentration that can be incorporated into the DNP matrix, while still achieving good DNP performance. This solubility threshold depends on the chemical properties of the glassing solvent of choice 3. Moreover, 129Xe magnetization throughput, reliable sample preparation and extraction of the hyperpolarized gas are still far from hyperpolarized 13C standards.
In this study, we tackle these weaknesses of the technique by means of a purpose-built sample preparation setup and the implementation of microwave frequency modulation, and study the benefits of these technical upgrades on the Xe sample’s properties.
Methods
The Xe DNP sample was prepared as follows: a sample vial filled with 200ul of 34mM TEMPO (2,2,6,6-Tetramethyl-1-piperidinyloxy) in isobutanol was pressurized with 8 bar of natural Xe, and immersed in a bath of melting ethanol to controllably reach -110°C, close to the triple point of Xe.Condensed liquid Xe was incorporated into the glassing solvent by applying 500W of 20kHz ultrasounds to the ethanol bath for 10 minutes by means of a ultrasonic processor.
This yielded a sample containing 3.25M Xe and 30mM TEMPO in isobutanol. To study the effect of ultrasonication on the DNP properties, a similar preparation was repeated without ultrasonication by incorporating the Xe using manual agitation.
The sample vial was then connected to a custom fluid path (CFP) 4 and loaded into the 5T/1.2K polarizer.
Both samples were characterized in the solid-state as follows:
· Microwave frequency sweeps (139.5-140.5GHz) without and with 100MHz of microwave frequency modulation (FM) to determine the DNP profiles and optimal polarization frequencies.
· DNP buildup: the samples were hyperpolarized at their positive DNP enhancement frequencies with and without microwave FM. The buildup was monitored every 2min by 5° RF pulses.
· Solid-state polarization measurements: the sample was thermally polarized during 6h and monitored every 30min by 10° pulses. The solid-state polarization was quantified by comparing the signals of both DNP and thermal buildups.
· Longitudinally-detected ESR (LOD-ESR): The longitudinal relaxation time of the radical’s electrons (T1e) was measured at DNP conditions using a custom-designed setup4.
Finally, the sonicated sample was hyperpolarized at the optimal settings to yield the maximal achievable polarization. After reaching the plateau, the Xe gas was extracted using 7.5ml of D2O at 180°C, and transferred to a pressurized 5mm tube placed inside a 1.05T benchtop NMR spectrometer (SpinSolve 43 13C/129Xe, Magritek) to measure the gas-state hyperpolarized signal with 5° pulses every 4.5s.
Results
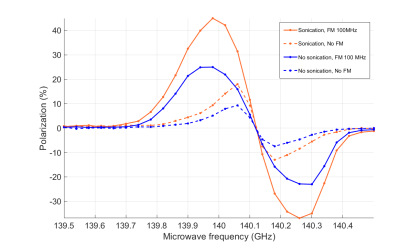
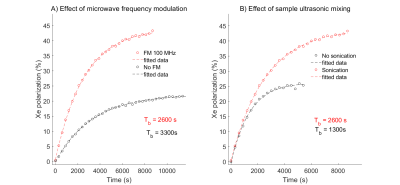
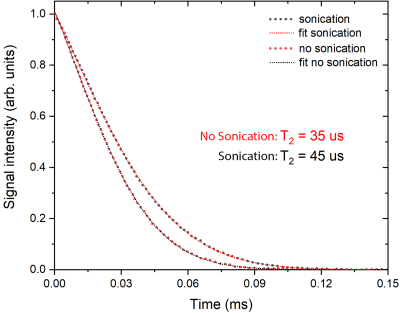
DNP profiles of both samples acquired are shown on Fig.1. Microwave FM efficiently and enhanced the polarization on both samples, leading to higher polarization levels and decreasing the buildup time (Fig.2A).Ultrasonic mixing did not have an effect on the initial polarization rate compared to manual mixing, but resulted in a substantially higher polarization level (Fig.2B). Furthermore, ultrasonic mixing resulted in a 29% longer apparent T2 in on the NMR FID signal (Fig.3).
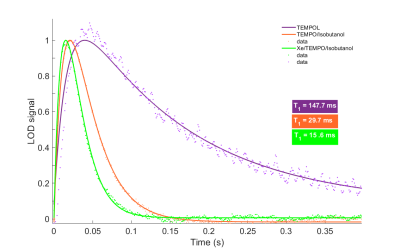
The radicals electronic properties of TEMPO were drastically influenced by the sample matrix. In isobutanol, the T1e was only 29.7ms compared to about 150ms in a classical water:glycerol matrix, and further halved by the addition of Xe to the sample (Fig.4).
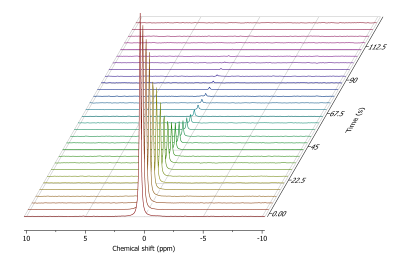
Xenon hyperpolarized via DNP could be successfully extracted and measured in the gas-state (Fig.5).
Discussion
The ultrasonication procedure yields a superior incorporation of the Xe atoms inside the solvent by improvement of the microscopic sample homogeneity, as confirmed by a 29% longer T2 when compared to samples prepared with the original procedure2,3. As it is well-known in dDNP, a more homogeneous sample often results in higher polarization5, which was observed in our measurements.An interesting observation came from the radical’s T1e measurements. Due to the low melting point of xenon (-112 °C), the sample matrix preparation involves short chain alcohols, isobutanol in the present case, leading to a short T1e. This value was further reduced after adding xenon to the matrix. In overall, the T1e of the DNP sample was ten-fold shorter compared to TEMPOL in a water:glycerol matrix that could be used for 13C DNP. As a consequence, efficient saturation of the ESR line becomes challenging for Xe DNP samples. Here, microwave frequency modulation represents a workaround and was used to promote spectral spin diffusion and boost the polarization.
Conclusion
Xe DNP is a challenging topic due to the extent of the different steps of the process, and their sensitivity to human error. Nevertheless, careful understanding of the physics and chemistry behind can not only boost the process to match SEOP standards, but also offers the chance to acquire deeper theoretical insight.Acknowledgements
This study is supported by the Swiss National Science Foundation (190547 and 193276 to A. Capozzi).References
[1] G. Norquay, G. J. Collier, M. Rao, et al. 129Xe-Rb Spin-Exchange Optical Pumping with High Photon Efficiency. Phys. Rev. Lett. 2018; 121
[2] A. Comment, S. Jannin, J.-N. Hyacinthe, et al. Hyperpolarizing Gases via Dynamic Nuclear Polarization and Sublimation. Phys. Rev. Lett, 2010; 105.
[3] A. Capozzi,C. Roussel,A. Comment, et al. Optimal Glass-Forming Solvent Brings Sublimation Dynamic Nuclear Polarization to 129Xe Hyperpolarization Biomedical Imaging Standards. Phys. Chem. C. 2015; 119
[4] A. Capozzi, J. Kilund, M. Karlsson, et al. Metabolic contrast agents produced from transported solid 13C-glucose hyperpolarized via dynamic nuclear polarization. Commun Chem, 2021; 4, 95
[5] A. Capozzi, M. Karlsson, J. Petersen, et.al. Liquid-State 13C Polarization of 30% through Photoinduced Nonpersistent Radicals. The Journal of Physical Chemistry C 2018 122 (13), 7432-7443
[6] Ji, X., Bornet, A., Vuichoud, B. et al. Transportable hyperpolarized metabolites. Nat Commun 2017; 8, 13975
Figures