1185
Parahydrogen-Induced Polarization Relayed via Proton Exchange: Application to biological molecules.1Section Biomedical Imaging, Molecular Imaging North Competence Center (MOIN CC), Department of Radiology and Neuroradiology, University Medical Center Schleswig-Holstein and Kiel University, Kiel, Germany
Synopsis
Parahydrogen-induced polarization relayed via proton exchange (PHIP-X) is a new method to hyperpolarize a variety of molecules that participate in suitable fast proton exchange with allyl alcohol. The combination of the wide applicability of polarization-transfer via proton exchange and the strong polarization obtained using hydrogenative of PHIP opens up new avenues to hyperpolarize biological molecules such as glucose, lactate and pyruvate. Hence, PHIP-X provides a promisingnew platform for potential applications in metabolic MRI.
Abstract
Introduction:Magnetic resonance imaging (MRI) with hyperpolarized contrast agents has enabled many new applications, including real time metabolic imaging.1 Despite this success, however, the cost for some hyperpolarization methods remains high, and not all agents can yet be polarized high enough. Here, we present a new method2 that allows polarizing many molecules undergoing proton exchange. Here, a transfer agent is polarized by adding parahydrogen, followed by transfer to a target via proton exchange (Fig. 1). This approach allows combining the low costs and strong hyperpolarization from hydrogenative parahydrogen-induced polarization3 (PHIP) with the broad applicability of polarization transfer via proton exchange4. 1H and 13C hyperpolairzation was observed.
Method:
Six different precursors (Fig. 2, 1 – 6) were purchased and solved in three different solvents (acetone-d6, dimethyl sulfoxide-d6 and dichloromethane-d2). Hyperpolarization was carried out by catalytic addition of 50 % pH2 using [Rh(dppb)(COD)]BF4 at 1 or 30 bar for 20 s in different different external magnetic fields. The hydrogenation at 1 bar was carried out in a 5mm NMR tube. The hydrogenation at 30 bar was carried out in a copper tube. After hydrogenation at low field the hyperpolarized solution was immediately transferred into a 1T NMR for detection (spinsolve, magritek). Polarization was quantified with respect to the sample in thermal equilibrium.
Results:
Transfer agent 1 in acetone-d6 provided the strongest polarizations and a sufficient hydrogenation yield (Fig. 3): P1H = 13 % was observed on H2C= after 20 s hydrogenation at only 1 bar with 50% enriched5 pH2 at 6 mT. Using 100 % pH2 is expected to triple the polarization. The exchanging OH was polarized to 4.2%. Already here, polarization transfer to residual water of the solvent and to unreacted precursor was observed.
When the experiment was repeated with a dedicated target, e. g. ethanol (Fig. 4), strong 1H polarization of 0.4% was observed. Some agents, however, did show lower polarizations. 13C-hyperpolarization was demonstrated using 43mM 13C6-glucose: here, 600-fold enhanced 13C signal was observed using a 90° excitation, without using a dedicated sequence to transfer the polarization of 1H to 13C (fig. 5).
A PHIP-X experiment with the very high concentration of 1,000 mM of 13C3-lactic acid in acetone-d6 provided 350-fold enhanced 13C signal when a DEPT sequence was used to transfer polarization from 1H to 13C.
Discussion:
PHIP-X was found to polarize many different targets within seconds, including water, lactic acid, pyruvic acid, glucose and various alcohols. Likely, many more molecules which participate in a suitable fast proton exchange with allyl alcohol are suitable for PHIP-X; not only molecules containing -OH groups, but -SH and -NH too. Challenges include that some targets were found to hinder the hydrogenation, that unreacted transfer agent my consume polarization, and that a higher polarization yields and biocompatible solvents are needed for a biomedical application. These items may be addressed by adding the target after hydrogenation, using 100 % pH2, higher pressure and better spin order transfers.
Conclusion:
PHIP-X is a promising method to generate a strong MR signal amplifications on a variety of target molecules, combining the high polarization obtained using hydrogenative PHIP with the broad applicability of polarization transfer via proton exchange.
Acknowledgements
We acknowledge support by the Emmy Noether Program“Metabolic and Molecular MR” (HO 4604/2-2), the researchtraining circle “Materials for Brain” (GRK 2154/1-2019),DFG-RFBR grant (HO 4604/3-1, No 19-53-12013), Clusterof Excellence “Precision Medicine in Inflammation” (PMI2167), German Federal Ministry of Education and Research(BMBF) within the framework of the e:Med research andfunding concept (01ZX1915C). Kiel University and theMedical Faculty are acknowledged for supporting theMolecular Imaging North Competence Center (MOIN CC,MOIN 4604/3). MOIN CC was founded by a grant from theEuropean Regional Development Fund (ERDF) and theZukunftsprogramm Wirtschaft of Schleswig-Holstein (Projectno. 122-09-053). The Russian team thanks the RussianFoundation for Basic Research (Grant 19-53-12013) for financial support. Additionally we thank Jule, Andrey and Frowin for interesting discussions.References
[1] Jan-Bernd Hövener et. al.: Parahydrogen-Based Hyperpolarization for Biomedicine. Angew Chem Int Ed Engl. 2018 Aug 27; 57(35): 11140–11162.
[2] Kolja Them, Frowin Ellermann, Andrey N. Pravdivtsev, Oleg G. Salnikov, Ivan V. Skovpin, Igor V. Koptyug, Rainer Herges, and Jan-Bernd Hövener: Parahydrogen-Induced Polarization Relayed via Proton Exchange. J. Am. Chem. Soc. 2021, 143, 34, 13694–13700.
[3] C. Russell Bowers and D. P. Weitekamp: Parahydrogen and synthesis allow dramatically enhanced nuclear alignment. J. Am. Chem. Soc. 1987, 109, 18, 5541–5542.
[4] Wissam Iali, Peter J. Rayner and Simon B. Duckett: Using parahydrogen to hyperpolarize amines, amides, carboxylic acids, alcohols, phosphates, and carbonates. Sci. Adv. 2018; 4: eaao6250.
[5] Frowin Ellermann, Andrey Pravdivtsev, and Jan-Bernd Hövener: Open-source, partially 3D-printed, high-pressure (50-bar) liquid-nitrogen-cooled parahydrogen generator. Magn. Reson., 2, 49–62, 2021.
Figures
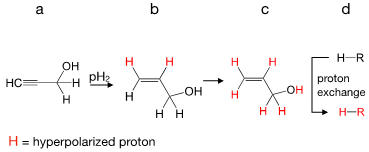
Figure 1: Schematic view of PHIP-X: pH2 is added to an unsaturated precursor (a) to form the transfer agent (b). At low magnetic fields, all protons acquire net polarization (c, red) that can be transferred to a receiver molecule (d) via proton exchange. Modified with permission from J. Am. Chem. Soc. 2021, 143, 34, 13694–13700. Copyright 2021 American Chemical Society.
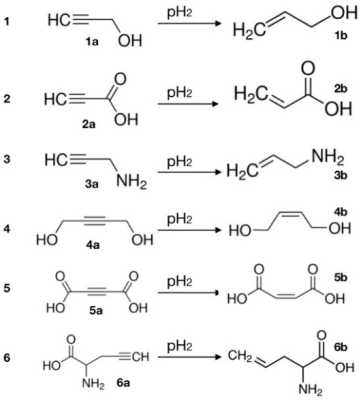
Figure 2: Structures of precursors (1a – 6a) and transfer agents (2b – 6b) tested for PHIP-X. Reprinted (adapted) with permission from J. Am. Chem. Soc. 2021, 143, 34, 13694–13700. Copyright 2021 American Chemical Society.
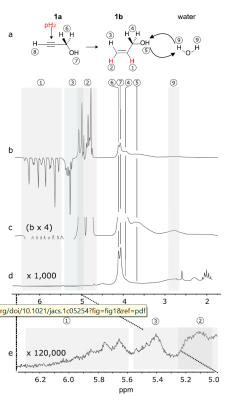
Figure 3: PHIP-X scheme and spectra of system 1 with water as a target: hydrogenation and proton relayed polarization exchange scheme (a), hyperpolarized (b, c) and thermal 1H NMR spectrum (d, e, 12,000 transients). Strong polarization was found on 1a, 1b, and H2O. Reprinted (adapted) with permission from J. Am. Chem. Soc. 2021, 143, 34, 13694–13700. Copyright 2021 American Chemical Society.
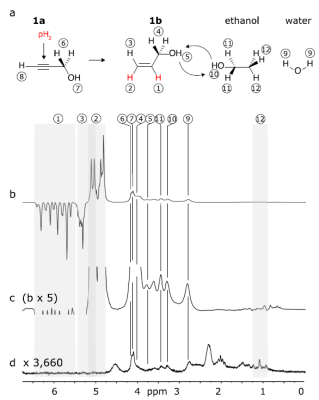
Figure 4: : PHIP-X of ethanol and water with system 1 in acetone-d6: polarization scheme (a), corresponding hyperpolarized (b, c) and thermal 1H NMR spectra (d, 50 transients). Strong polarization was observed for 1a, 1b, H2O and ethanol. Reprinted (adapted) with permission from J. Am. Chem. Soc. 2021, 143, 34, 13694–13700. Copyright 2021 American Chemical Society.
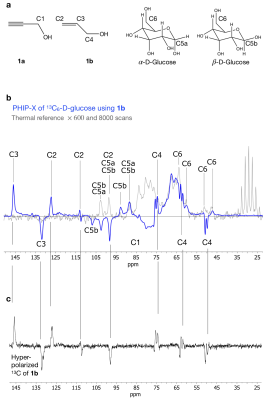
Figure 5: PHIP-X of 13C6-D-glucose. Molecular structures with indicated 13C nuclei of 1b and glucose (a). 13C PHIP-X spectrum of 46 mM hyperpolarized 13C6-D-glucose (b, blue), thermal spectrum of 46 mM glucose (b, grey), and hyperpolarized 1b without glucose (c). Reprinted (adapted) with permission from J. Am. Chem. Soc. 2021, 143, 34, 13694–13700. Copyright 2021 American Chemical Society.