1180
OxyChip embedded with gold nanoparticles as a sensor for EPR oximetry and self-fiducial for anatomic registration
Maciej M Kmiec1, Kendra A Hebert1, Dan Tse1, Sassan Hodge1, Benjamin B Williams2, Philip E Schaner2, and Periannan Kuppusamy3
1Geisel School of Medicine at Dartmouth College, Lebanon, NH, United States, 2Medicine, Dartmouth-Hitchcock Medical Center, Lebanon, NH, United States, 3Radiology, Geisel School of Medicine at Dartmouth College, Lebanon, NH, United States
1Geisel School of Medicine at Dartmouth College, Lebanon, NH, United States, 2Medicine, Dartmouth-Hitchcock Medical Center, Lebanon, NH, United States, 3Radiology, Geisel School of Medicine at Dartmouth College, Lebanon, NH, United States
Synopsis
EPR oximetry using the OxyChip can provide direct and repeated measurement of tissue oxygen; however, a significant limitation is its inability to identify the location of the probe in the tissue. We report the development of an OxyChip embedded with gold nanoparticles to visualize the implant using clinical ultrasound or CT. In vitro characterization, imaging, and histopathology of the probe using tissue phantoms, excised tissues, and in vivo animal models demonstrated a substantial enhancement of CT contrast with OxyChip-GNP without compromising its oxygen-sensing properties or biocompatibility. The OxyChip-GNP can facilitate precisely localized in vivo oxygen measurements in the clinical setting.
Introduction
Electron paramagnetic resonance (EPR) oximetry using the OxyChip as an implantable oxygen sensor can directly and repeatedly measure tissue oxygen levels (pO2)1-4. The OxyChip is fabricated by embedding oxygen-sensitive microcrystals of LiNc-BuO in PDMS polymer1. It is typically implanted by placing it inside the needle and deploying it into the tissue. A recent first-in-human clinical study from our team has established the safety and feasibility of the OxyChip for reliable and repeated pO2 measurements in a variety of tumors and treatment regimens5,6. A notable limitation in these studies is the inability to easily locate and identify the implanted probes in the tissue, particularly in the long term, thus limiting spatial/anatomical registration of the implant for proper interpretation of the oxygen data. Reliable localization of the OxyChip to the area of interest (i.e., the tumor itself) is critical to the success of repeated hypoxia assessments and subsequent targeted treatments. Thus, it is clearly desirable to have a high degree of confidence that the OxyChip is placed within the tumor and that its location can be easily verified during and after placement. In addition, the position of the OxyChip relative to regions of the tumor responding to treatment identified by anatomical and functional imaging will be helpful in addressing the impacts of oxygen heterogeneity in the tumor. Currently, the OxyChip is not radiopaque, and it is also difficult to visualize by routine clinical imaging modalities, such as ultrasound or computed tomography (CT). It is thus challenging to locate the OxyChip during EPR measurements or prior to or during surgery.Purpose
The purpose of this study was to develop the OxyChip with enhanced CT contrast by embedding gold nanoparticles (GNP) to enable visualization of the implant using routine clinical imaging modalities such as CT.Methods
The OxyChip was fabricated by cast-molding polymerization of polydimethylsiloxane (PDMS) in the presence of LiNc-BuO microcrystals1. GNPs were embedded by incubation of the prefabricated OxyChip with a 10% solution (w/v) of gold (III) chloride in 100% ethanol for 72 hours. In vitro and in vivo characterization, scanning electron microscopy, ultrasound, micro/clinical CT, EPR oximetry, and histopathology studies were carried out using tissue phantoms, excised tissues, and in vivo animal models (mice and rats). Contrast enhancement of the OxyChip-GNP was compared with OxyChip and standard clinical fiducials, PolyMark and solid gold, expressed in HU (Hounsfield units).Results
SEM and X-ray energy-dispersive spectroscopy revealed the diffusion-limited distribution of GNPs inside the OxyChip (Fig. 1). The embedding of GNPs in the OxyChip did not have any significant effect on the EPR properties of the OxyChip: EPR sensitivity; linear dependence of EPR signal width with pO2; and response time to dynamic changes of oxygen level. The oxygen-sensing ability of the OxyChip-GNP was unaffected by exposure to 60-Gy high-energy radiation or sterilization by autoclaving. Evaluation of the OxyChip-GNP implanted in the leg muscle of rats for 30 days revealed no changes to its oxygen sensitivity. We further evaluated the CT contrast of OxyChip-GNP using micro-CT in chicken meat , which demonstrated significant contrast enhancement in the OxyChip-GNP compared to the bare OxyChip (Fig. 2). The OxyChip-GNP was clearly visualized and localized by CT and CB-CT in the clinical setting (Fig. 3). Repeated measurements of pO2 in the leg muscle of mice obtained over a 10-week period suggested that GNP embedding has no significant effect on the in vivo oxygen measurement capability of the OxyChip-GNP (Fig. 4). Histopathological examination of the implant site (tissue) showed moderate fibrosis surrounding the insertion track after 3 weeks, and no fibrosis or inflammatory reaction after 3 months.Discussion
The results of the present study demonstrated the clinical utility of the OxyChip-GNP for oxygen measurement and identification. Incorporation of GNPs in preformed OxyChips did not compromise their EPR and oxygen-sensing properties or biocompatibility. The results also showed a substantial enhancement of ultrasound and CT contrast relative to the standard OxyChip, allowing for easy visualization of the OxyChip-GNP and demonstrating its potential for use facilitating anatomic registration of the implant. The CT contrast enhancement of the OxyChip-GNP is adequate for its in vivo identification using a clinical CT scanner as well as a CB-CT scanner. This is important, as it allows for the potential for daily visualization of the OxyChip-GNP during radiotherapy using daily CB-CT, thereby providing critical information about the anatomy being evaluated via tissue oximetry. In this regard, the OxyChip-GNP allows visualization of its location in addition to providing oxygen data at the site of implant. Visualization of the OxyChip-GNP may also allow it to serve as a traditional fiducial marker, enabling x-ray localization of soft-tissue structures during treatment or prior to surgical resection. This development is highly significant in that repeated in vivo measurements of tissue pO2 with precise anatomic localization enhances our ability to accurately interpret the pO2 data obtained.Conclusion
The OxyChips embedded with gold nanoparticles (OxyChip-GNP) can be readily identified in soft tissues using standard clinical imaging modalities such as CT, CB-CT, or ultrasound imaging while maintaining its capability to make repeated in vivo measurements of tissue oxygen levels over the long term. This unique capability of the OxyChip-GNP facilitates precisely localized in vivo oxygen measurements in the clinical setting.Acknowledgements
The study was supported by National Institutes of Health grants R01 EB004031 and P01 CA190193.References
- Hou H, Khan N, Gohain S, et al. Pre-clinical evaluation of OxyChip for long-term EPR oximetry. Biomed Microdevices, 2018. 20(2): p. 29.
- Kmiec MM, Hou H, Kuppusamy ML, et al. Transcutaneous oxygen measurement in humans using a paramagnetic skin adhesive film. Magn Reson Med, 2019. 81(2): p. 781-794.
- Kmiec MM, Tse D, Kuppusamy, P. Oxygen-Sensing Paramagnetic Probes for Clinical Oximetry. Adv Exp Med Biol, 2021. 1269: p. 259-263.
- Kmiec MM, Tse D, Mast JM, et al. Implantable microchip containing oxygen-sensing paramagnetic crystals for long-term, repeated, and multisite in vivo oximetry. Biomed Microdevices, 2019. 21(3): p. 71.
- Schaner PE, Pettus JR, Flood AB, et al. OxyChip Implantation and Subsequent Electron Paramagnetic Resonance Oximetry in Human Tumors Is Safe and Feasible: First Experience in 24 Patients. Front Oncol, 2020. 10: p. 572060.
- Schaner PE, Williams BB, Chen EY, et al. First-In-Human Study in Cancer Patients Establishing the Feasibility of Oxygen Measurements in Tumors Using Electron Paramagnetic Resonance With the OxyChip. Front Oncol, 2021. 11: p. 743256.
Figures
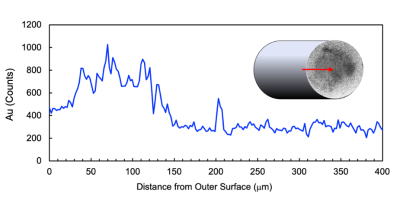
Fig.1 X-ray energy-dispersive spectroscopy of gold (Au) along a line-scan in the middle of cross-sectional surface of OxyChip-GNP (0.6-mm diameter) as shown in the SEM image at 350x magnification in the inset. The red arrow indicates the position and direction of X-ray analysis. The data indicate a progressively decreasing level of gold with the highest density at about 100-micron depth.
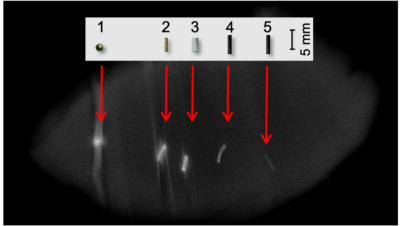
Fig. 2 Micro-CT image comparing OxyChips/GNP and clinical fiducials. The OxyChips/GNP and fiducials were implanted in a chicken meat and imaged using a micro-CT scanner (eXplore Locus Scanner, GE Healthcare). Legend: (1) Solid gold sphere (bone fiducial); (2) Solid gold rod (soft tissue fiducial); (3) PolyMark™ fiducial (soft tissue fiducial); (4) OxyChip-GNP; (5) OxyChip (without GNP).
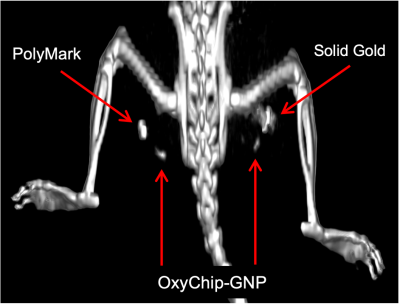
Fig. 3 Clinical CB-CT image comparing OxyChip-GNP with standard fiducials. Two OxyChip-GNPs and two clinical fiducials (PolyMark and solid gold) were implanted in the rat leg muscle. The image, which was obtained using CB-CT scanner (Varian Medical System - TrueBeam), reveals both the OxyChip-GNPs along with the clinical fiducials.
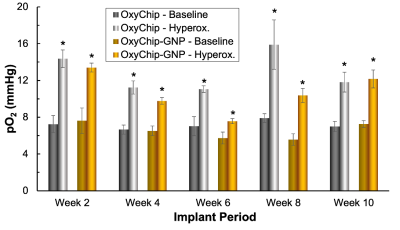
Fig. 4 pO2 values measured in the leg muscle of mice using OxyChip-GNP. Mean pO2 values of baseline and during hyperoxygen-breathing (100% O2 for 20 min) obtained biweekly from the OxyChip and OxyChip-GNP *P<0.05 - significantly higher compared to respective baseline value. Analysis of the baseline and hyperoxygen data showed a significant increase of hyperoxygen pO2 compared to baseline (OxyChip, P=0.0018; OxyChip-GNP, P=0.0042) suggesting that GNP embedding has no significant effect on the in vivo oxygen measurement capability.
DOI: https://doi.org/10.58530/2022/1180