1179
Tumor oxygen levels and their response to hyperoxygenation in cancer patients measured using EPR oximetry with the OxyChip
Periannan Kuppusamy1, Benjamin B Williams2, Eunice Y Chen3, Maciej M Kmiec4, and Philip E Schaner2
1Radiology, Geisel School of Medicine at Dartmouth College, Lebanon, NH, United States, 2Medicine, Dartmouth-Hitchcock Medical Center, Lebanon, NH, United States, 3Dartmouth-Hitchcock Medical Center, Lebanon, NH, United States, 4Geisel School of Medicine at Dartmouth College, Lebanon, NH, United States
1Radiology, Geisel School of Medicine at Dartmouth College, Lebanon, NH, United States, 2Medicine, Dartmouth-Hitchcock Medical Center, Lebanon, NH, United States, 3Dartmouth-Hitchcock Medical Center, Lebanon, NH, United States, 4Geisel School of Medicine at Dartmouth College, Lebanon, NH, United States
Synopsis
We report a first-in-humans clinical study of EPR oximetry using the OxyChip to establish its feasibility and utility for clinically useful tumor oxygen measurements in cancer patients. Repeated measurements from a cohort of 11 cancer patients in 33 sessions over a long period of time revealed variable levels of clinically significant hypoxia as well as variable responses to a hypoxia-mitigation intervention. Overall, in light of this variability, this study further underscores the need to provide individualized repeatable assessment of tumor oxygenation in the context of planned hyperoxygenation interventions to optimize clinical outcomes.
Introduction
Most solid tumors contain regions of acute and chronic hypoxia that can negatively impact treatment outcomes in cancer patients. Oxygen level in solid tumors is a critical parameter that particularly affects radiation therapy, as hypoxia increases resistance to treatment. Recent clinical trials, e.g., the ARCON trial1 emphasized the need for a stratification of the patient population with respect to tumor hypoxia in order to optimize treatment outcomes. Therefore, it is highly desirable to monitor oxygen levels in tumors before and during therapy. This would require the availability of a means to make reliable, repeated, and direct measurements of oxygen levels in tumors at specific anatomical locations. Several invasive and noninvasive methods exist for tumor oximetry; however, a suitable method for direct and repeated measurements of tumor oxygen in the clinical setting has not been established. In vivo electron paramagnetic resonance (EPR) oximetry can make clinically relevant dynamic measurements including the unique ability to perform continuous direct detection of absolute levels of oxygen, at significant depths and with repeated measurements over time2,3.Objective
The overall objective of this first-in-humans clinical study was to establish EPR oximetry using an implantable oxygen sensor, called the ‘OxyChip’,2 as a clinically feasible technology that would allow individualized tumor-oxygen assessments in cancer patients prior to and during hypoxia-modification interventions such as hyperoxygen breathing.Methods
Patients with any type of solid tumor at ≤3-cm depth from the skin-surface scheduled to undergo surgical resection (with or without neoadjuvant therapy) were eligible for the study4. The OxyChip was implanted in the tumor and subsequently removed during standard-of-care surgery5. Partial pressure of oxygen (pO2) at the implant location was measured using EPR oximetry with a custom-built clinical EPR scanner operating at 1.15 GHz. During each measurement session, the patient would breathe room air, followed by a period of oxygen inhalation using a non-rebreather mask with 100% oxygen, and then breathe room air again. The location of the OxyChip in the tumor was confirmed either during ultrasound-guided implantation and/or at tumor resection5.Results
EPR oximetry was carried out in the tumors of 11 cancer patients (squamous cell carcinoma of the skin 4; breast cancer 3; basal cell carcinoma 2; melanoma 2) over a total of 33 visits. Fig. 1 shows measurements repeatedly carried out in a breast cancer patient undergoing chemotherapy for >4 months. Of the 33 total measurements, the mean of tumor pO2 values was 16.3±2.9 mmHg (median 12.4 mmHg, range 0.6 to 73.1 mmHg), while the mean of hyperoxygen values was 36.4±6.6 mmHg (median 20.5 mmHg, range 1.5–144.6 mmHg). A pair-wise representation of base and hyperoxygen values from these measurements is shown in Fig. 2A. The radiobiological response of cancers to tumor oxygenation was determined in the form of oxygen enhancement ratio (OER) as reported6. Fig. 2B shows a view of the level of radio-sensitization, in terms of OER, by hyperoxygenation. The results indicated that in about 27% of the measurements (9 out of 33) the tumors were severely hypoxic (pO2<5 mmHg) and 67% (6 out of 9) of them could be sensitized to radiation with ≥20% OER gain. Further, about 42% of the values (14 out of 33) were radiobiologically hypoxic (pO2<10 mmHg) and 64% (9 out of 14) of the hypoxic tumors could be sensitized to radiation with ≥10% OER gain. Twenty-four measurements (73%) showed the tumors were not severely hypoxic (pO2≥5 mmHg) and, irrespective of whether they responded or not to hyperoxygenation, increased radiosensitization according to the OER model would be limited to <20%. Overall, these results emphasize the need to measure pO2 in each tumor at the time of treatment to optimize hypoxia-mitigation strategies.Discussion
The results of the present study establish, for the first time, that tumor oxygen levels can be measured in cancer patients repeatedly using EPR oximetry with the OxyChip to obtain both initial baseline pO2 values and values after interventions designed to increase tumor oxygenation. Importantly, these results also demonstrate the capacity to measure these values successfully over long periods of time. The oxygen data obtained from these patients showed considerable variations among tumors as well as in the same tumor, with or without cancer-directed therapy, as a function of time. Clinically significant hypoxia was observed in a subset of tumors with varying levels of response to hypoxia-mitigation by breathing oxygen-enriched gas. However, despite the significant heterogeneity of tumor pO2, we observed statistically significant increases in tumor oxygen following administration of hyperoxygenated gas across various types of tumors and patients. This finding suggests that the OxyChip has the potential to measure subtle changes of tumor oxygen before and during cancer treatment and as such paves the way for using EPR oximetry in the clinical setting for cancer prognosis and treatment planning.Conclusion
Measurement of baseline pO2 and response to hyperoxygenation using EPR oximetry with the OxyChip is clinically feasible in a variety of tumor types. Tumor oxygen at baseline differed significantly among patients. Although most tumors responded to a hyperoxygenation intervention, some were non-responders. These data demonstrated the need for individualized assessment of tumor oxygenation in the context of planned hyperoxygenation interventions to optimize clinical outcomes.Acknowledgements
The study was supported by National Institutes of Health grants R01 EB004031 and P01 CA190193.References
- Janssens GO, Rademakers SE, Terhaard CH, et al. Accelerated radiotherapy with carbogen and nicotinamide for laryngeal cancer: results of a phase III randomized trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 2012. 30(15): p. 1777-1783.
- Hou H, Khan N, Gohain S et al. Pre-clinical evaluation of OxyChip for long-term EPR oximetry. Biomed Microdevices, 2018. 20(2): p. 29.
- Kmiec MM, Hou H, Kuppusamy ML, et al. Transcutaneous oxygen measurement in humans using a paramagnetic skin adhesive film. Magn Reson Med, 2019. 81(2): p. 781-794.
- Schaner PE, Williams BB, Chen EY et al. First-In-Human Study in Cancer Patients Establishing the Feasibility of Oxygen Measurements in Tumors Using Electron Paramagnetic Resonance With the OxyChip. Front Oncol, 2021. 11: p. 743256.
- Schaner PE, Pettus JR, Flood AB et al. OxyChip Implantation and Subsequent Electron Paramagnetic Resonance Oximetry in Human Tumors Is Safe and Feasible: First Experience in 24 Patients. Front Oncol, 2020. 10: p. 572060.
- Grimes DR, Partridge M. A mechanistic investigation of the oxygen fixation hypothesis and oxygen enhancement ratio. Biomed Phys Eng Express, 2015. 1(4): p. 045209.
Figures
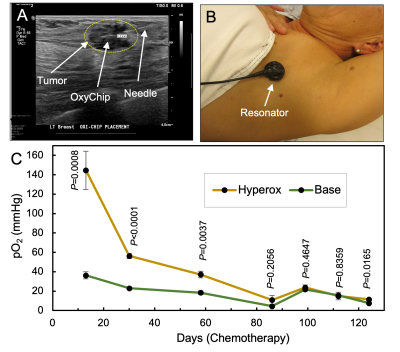
Fig. 1 OxyChip implantation, EPR measurement, and pO2 values in a malignant left axillary node from a breast cancer in a 55-year-old female undergoing chemotherapy. (A) Ultrasound-guided implantation of the OxyChip using a brachytherapy needle. The OxyChip has been deployed within the tumor, and the needle is being retracted. (B) EPR measurement using a flexible surface-coil detector placed over the tumor. (C) Changes in pO2 (baseline and response to hyperoxygenation; mean±SEM) during the treatment period. P values are in comparison to the corresponding base pO2 values.
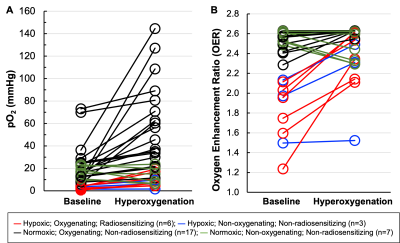
Fig. 2 Mitigation of tumor hypoxia for therapeutic enhancement. (A) Pair-wise representation of the base and hyperoxygen pO2 values (33 sets from 11 patients). (B) Level of radio-sensitization, in terms of oxygen enhancement ratio (OER), by hyperoxygenation. Nine measurements had severely hypoxic (pO2<5 mmHg) tumors of which 6 could be sensitized to radiation (i.e., ≥20% OER gain). Twenty-four measurements had pO2≥5 mmHg and, irrespective of whether they responded or not, hyperoxygenation probably would not have had a beneficial radio-sensitizing effect at the times measured.
DOI: https://doi.org/10.58530/2022/1179