1174
Age and lung volume dependence of dissolved xenon-129 imaging parameters1POLARIS, University of Sheffield, Sheffield, United Kingdom, 2GE Healthcare, Munich, Germany
Synopsis
This work investigates the correlation between age and pulmonary gas transfer measurements assessed with dissolved xenon-129 MRI in 26 healthy volunteers with an age range of 23 to 68yrs. Dependence on lung inflation is also investigated by performing imaging at total lung capacity. Results show significant negative correlations of xenon tissue uptake and blood transfer and suggest that an age-correction should be performed when reporting results. Imaging at total lung capacity also confirms a significant decrease in xenon gas transfer when compared to imaging during standard inspiratory breath-hold.
Introduction
Due to the large chemical shift separation of 129Xe gas, 129Xe dissolved in lung tissue/plasma (TP) and in red blood cells (RBC), it is possible to acquire spectroscopic images of gas transfer by calculating ratio maps of 129Xe signal in the different compartments. These metrics have shown sensitivity to regional gas transfer limitation and disease progression in interstitial and obstructive lung diseases1-3. However, normative data sets from healthy subjects across broad age ranges are also needed before wide-ranging clinical conclusions can be drawn. RBC:TP, tissue uptake (TP:GAS) and blood transfer (RBC:GAS) ratios depend on sequence parameters such as the flip angle and repetition time (TR)4. An attempt to harmonize methods to acquire HP 129Xe lung MRI has been published recently5 with the goal of reducing inter-site variability of the measurements. However, little has been published on the effects of lung inflation and age dependence on the gas transfer ratios. Age and lung volume dependence of gas transfer are known physiological phenomena6 that have also been demonstrated in CSSR experiments using HP 129Xe7. Gas uptake and dissolved xenon imaging results are also subject to lung volume8 and dosing the inhaled gas according to subject’s height or total lung capacity has been recommended5. In this study, 26 healthy subjects across an age range of 23 to 68yrs were imaged at functional residual capacity plus a standard 1L bag (FRC+1L) and at total lung capacity (TLC) to evaluate the influence of age and lung volume on dissolved xenon imaging measurements.Methods
26 healthy volunteers with no known respiratory conditions were enrolled. Imaging was performed on a 1.5T GE HDx scanner while enriched 129Xe gas was polarized to ~30% with a spin-exchange optical pumping polariser (POLARIS, Sheffield, UK)9. FRC+1L images were acquired with a flexible quadrature transmit/receive vest coil during breath-hold after the inhalation of a bag of pure HP 129Xe from FRC. Images at TLC were acquired in 23 of the volunteers following the same initial procedure followed by inhalation of room air up to TLC. All subjects were taller than 160cm and received the maximum dose of 1L. An updated version of the 4-echo flyback 3D radial spectroscopic imaging technique described in 10 was used to image xenon gas, TP and RBC and create ratio maps of RBC:TP, RBC:GAS and TP:GAS. The main sequence improvements are as follows: (i) implementing a frequency-tailored RF excitation pulse to deliver a gas phase flip angle 1% of that delivered on tissue and blood, (ii) removing the interleaved RF excitation between gaseous and dissolved phase; (iii) new TR (15ms) and flip angle (22º) to match 5 and 934 radial projections; (iv) inserting the calibration spectrum required to estimate the resonance frequencies and T2* values at the start of the imaging sequence. In addition, the amplitude of the cardiogenic oscillation of the RBC signal (ARBCCO) during the 14s breath-hold was also derived11. Spearman correlations were computed between the dissolved 129Xe imaging markers and age of the subject. Wilcoxon paired t-tests were performed to study the differences between imaging markers at FRC+1L and TLC.Results
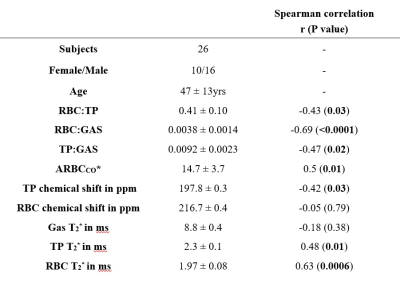
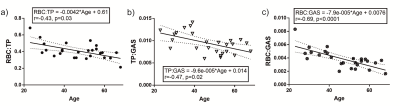
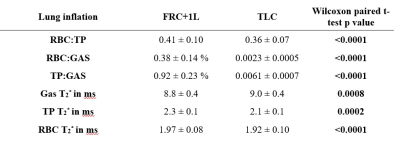
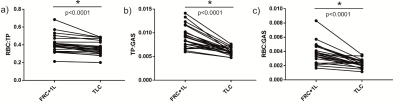
Table 1 summarizes the subject demographics and correlations of the dissolved xenon imaging markers with age. Although each global gas transfer ratio was significantly correlated with age, the strongest correlation was for the RBC:GAS transfer, showing a decline from 0.006 to 0.0021 between the ages of 20 and 70yrs (Figure 1). RBC:TP, the most-commonly reported dissolved xenon gas exchange imaging parameter, fell from 0.53 to 0.32 within this age range. All imaging markers were significantly different at TLC (Table 2 and Figure 2). The RBC:TP was reduced by a mean (SD) of 10.7±7.6% from FRC+1L to TLC whereas TP:GAS and RBC:GAS were reduced by 32±15% and 38±17% respectively. RBC:TP had the lowest average change (and SD) with lung inflation making it less susceptible to measurement error due to incorrect breathing manoeuvre.Discussion
The age dependence of dissolved xenon imaging measurements, previously observed with increased septal thickness in CSSR experiments7,12 has been confirmed, with a significant decrease of gas transfer, increase of ARBCCO, TP chemical shift and RBC T2* and a reduced RBC chemical shift in older healthy volunteers. With 129Xe lung MRI entering clinical practice13, and conclusions about lung pathophysiology, disease progression and treatment response being drawn from these ratios, it is important to have well-defined healthy reference values when making a clinical decision. Our results suggest that an age correction factor should be applied to 129Xe gas exchange ratios similar to standard pulmonary function tests. Regarding imaged lung volume, our results confirm that the volume of administered Xenon should be titrated according to subject lung volume and in addition, coaching to ensure that imaging is performed at a controlled lung inflation is necessary to minimize potential measurement errors.Conclusion
Dissolved 129Xe MRI gas transfer metrics in healthy volunteers depend upon age and lung inflation level. 129Xe gas transfer decreased with age and at total lung capacity suggesting that age correction and lung inflation level control are necessary for the correct interpretation of these metrics.Acknowledgements
This work was supported by MRC grant MR/M008894/1References
1. Wang JM, Robertson SH, Wang Z, He M, Virgincar RS, Schrank GM, Smigla RM, O'Riordan TG, Sundy J, Ebner L, Rackley CR, McAdams P, Driehuys B. Using hyperpolarized 129Xe MRI to quantify regional gas transfer in idiopathic pulmonary fibrosis. Thorax 2017.
2. Weatherley ND, Stewart NJ, Chan HF, Austin M, Smith LJ, Collier G, Rao M, Marshall H, Norquay G, Renshaw SA, Bianchi SM, Wild JM. Hyperpolarised xenon magnetic resonance spectroscopy for the longitudinal assessment of changes in gas diffusion in IPF. Thorax 2019;74(5):500-502.
3. Qing K, Tustison NJ, Mugler JP, 3rd, Mata JF, Lin Z, Zhao L, Wang D, Feng X, Shin JY, Callahan SJ, Bergman MP, Ruppert K, Altes TA, Cassani JM, Shim YM. Probing Changes in Lung Physiology in COPD Using CT, Perfusion MRI, and Hyperpolarized Xenon-129 MRI. Acad Radiol 2019;26(3):326-334.
4. Ruppert K, Amzajerdian F, Hamedani H, Xin Y, Loza L, Achekzai T, Duncan IF, Profka H, Siddiqui S, Pourfathi M, Sertic F, Cereda MF, Kadlecek S, Rizi RR. Assessment of flip angle-TR equivalence for standardized dissolved-phase imaging of the lung with hyperpolarized 129Xe MRI. Magn Reson Med 2018.
5. Niedbalski PJ, Hall CS, Castro M, Eddy RL, Rayment JH, Svenningsen S, Parraga G, Zanette B, Santyr GE, Thomen RP, Stewart NJ, Collier GJ, Chan HF, Wild JM, Fain SB, Miller GW, Mata JF, Mugler JP, 3rd, Driehuys B, Willmering MM, Cleveland ZI, Woods JC. Protocols for multi-site trials using hyperpolarized (129) Xe MRI for imaging of ventilation, alveolar-airspace size, and gas exchange: A position paper from the (129) Xe MRI clinical trials consortium. Magn Reson Med 2021.
6. Stanojevic S, Graham BL, Cooper BG, Thompson BR, Carter KW, Francis RW, Hall GL, Global Lung Function Initiative Twg, Global Lung Function Initiative T. Official ERS technical standards: Global Lung Function Initiative reference values for the carbon monoxide transfer factor for Caucasians. Eur Respir J 2017;50(3).
7. Stewart NJ, Leung G, Norquay G, Marshall H, Parra-Robles J, Murphy PS, Schulte RF, Elliot C, Condliffe R, Griffiths PD, Kiely DG, Whyte MK, Wolber J, Wild JM. Experimental validation of the hyperpolarized (129) Xe chemical shift saturation recovery technique in healthy volunteers and subjects with interstitial lung disease. Magn Reson Med 2015;74(1):196-207.
8. Hahn AD, Kammerman J, Evans M, Zha W, Cadman RV, Meyer K, Sandbo N, Fain SB. Repeatability of regional pulmonary functional metrics of Hyperpolarized (129) Xe dissolved-phase MRI. J Magn Reson Imaging 2019;50(4):1182-1190.
9. Norquay G, Collier GJ, Rao M, Stewart NJ, Wild JM. 129Xe-Rb Spin-Exchange Optical Pumping with High Photon Efficiency. Physical Review Letters 2018;121(15):153201.
10. Collier GJ, Eaden JA, Hughes PJC, Bianchi SM, Stewart NJ, Weatherley ND, Norquay G, Schulte RF, Wild JM. Dissolved (129) Xe lung MRI with four-echo 3D radial spectroscopic imaging: Quantification of regional gas transfer in idiopathic pulmonary fibrosis. Magn Reson Med 2021;85(5):2622-2633.
11. Bier EA, Robertson SH, Schrank GM, Rackley C, Mammarappallil JG, Rajagopal S, McAdams HP, Driehuys B. A protocol for quantifying cardiogenic oscillations in dynamic (129) Xe gas exchange spectroscopy: The effects of idiopathic pulmonary fibrosis. NMR Biomed 2019;32(1):e4029.
12. Ruppert K, Qing K, Patrie JT, Altes TA, Mugler JP, 3rd. Using Hyperpolarized Xenon-129 MRI to Quantify Early-Stage Lung Disease in Smokers. Acad Radiol 2019;26(3):355-366.
13. Stewart NJ, Smith LJ, Chan HF, Eaden JA, Rajaram S, Swift AJ, Weatherley ND, Biancardi A, Collier GJ, Hughes D, Klafkowski G, Johns CS, West N, Ugonna K, Bianchi SM, Lawson R, Sabroe I, Marshall H, Wild JM. Lung MRI with hyperpolarised gases: current & future clinical perspectives. Br J Radiol 2021:20210207.
Figures