1162
Brain MRI acceleration with deep modular networks (BRACELET)1Dept. of Medical Physics, Memorial Sloan Kettering Cancer Center, New York, NY, United States, 2Dept. of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY, United States
Synopsis
Brain cancer screening utilizing MRI suffers from a bottleneck by requiring 3D acquisitions before and after contrast injection. We designed a novel two-stage deep learning reconstruction pipeline to accelerate 3D brain MRI over conventional 2-fold parallel imaging accelerations used in clinical practice. Using modular deep neural networks, we removed the dependence on one all-encompassing network. We successfully dealiased brain images at higher accelerations and with structural fidelity in lesions superior to conventional clinical imaging. Our method was validated on pathologies unseen during training using qualitative evaluation from an expert neuroradiologist and achieved comparable scores to conventional 2-fold clinical accelerations.
Introduction
Magnetic resonance imaging (MRI) is a noninvasive and non-ionizing diagnostic modality that has shown immense utility for cancer detection and screening. However, MR physics create a bottleneck in the time required to acquire sufficient data. Consequently, MRI examination of brain tumors is significantly long as it requires 3D acquisitions before and after contrast injection with high spatial resolution. Accelerated approaches have leveraged advances in deep learning (DL) to produce substantial increases in acceleration factors. 1-5 In this study, we propose a novel DL reconstruction framework, BRACELET (Brain MRI ACceleration with dEep moduLar nETworks), to accelerate 3D brain MRI over conventional 2-fold parallel imaging accelerations used in clinical practice. BRACELET is trained using fully-sampled k-space data acquired on patients with different brain tumors and validated on unseen pathologies using qualitative evaluation from an expert neuroradiologist.Methods
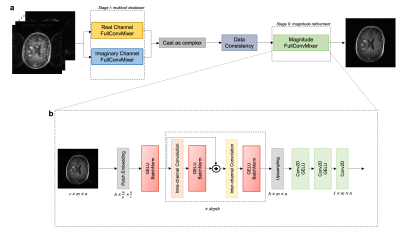
51 fully-sampled 3D T1-weighted scans were acquired using the BRAVO pulse sequence on 3T scanners (MR750W GE Healthcare). Scans were retrospectively accelerated along the ky and kz directions using a 2-by-2 Cartesian regular undersampling mask. Geometric coil compression 6 was performed to create uniform input data. To relax computational complexity, datasets were Fourier transformed along the fully-sampled readout direction and the resulting coronal slices were treated as individual samples.Recent DL computer vision architectures have featured operations on non-overlapping patches. 7-9 This allows for greater specificity to local structures within images while simultaneously enabling larger receptive fields (e.g., larger convolutional kernels). We chose a newly put forward method, ConvMixer, primarily because of its structural simplicity and comparable performance against higher complexity models while maintaining modest parameter counts. 9 ConvMixer originally is a classification model, so we modified it to be fully convolutional, and appropriately dubbed this model FullConvMixer (Figure 1).
Our philosophy was to stray from the conventional approach of designing one all-encompassing network. We instead aimed to devise a pipeline that enables differential control over focused modular components. This would allow varying the implicit importance of each component (i.e., allotted training time). For cancer screening, particular attention should be paid to lesion conspicuity and structural fidelity, rather than solely global image quality. Therefore, we hypothesized that training a network to refine a single-coil magnitude image would better aid clinical analysis.
Following this plan, we designed a two-stage reconstruction pipeline. Stage I performed multicoil dealiasing; stage II performed magnitude refinement. In stage I, we trained purely real- and imaginary-channel networks in parallel. 10 Data consistency (DC) was applied between stages. A neuroradiologist blindly scored each reconstruction on various qualitative metrics.
Results
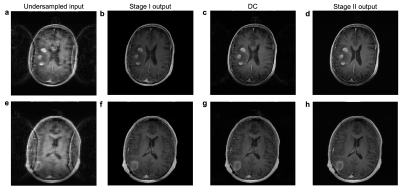
The two stages within BRACELET were trained independently. Each model had ~450k parameters, totaling only 1.5M parameters across three models. Due to differing intensity brightness profiles between the undersampled input images and the fully-sampled targets, the dealiasing networks learned dull intensity profiles. DC applied to the output improves the contrast between different tissues but reinjects subtle aliasing artifacts and grainy noise (Figure 2). Additionally, it highlights finer aliasing artifacts missed by stage I. Magnitude images are of primary importance to radiologists, so stage I + DC single-coil magnitudes were used as stage II magnitude refinement inputs.During stage I training, we observed that FullConvMixer removed macro aliasing artifacts after only a handful of epochs. Consequently, we trained stage II on early-stopped reconstructions. Figure 2 shows BRACELET results at every stage for 4-fold acceleration of two patient cases. The magnitude refinement model successfully removed remaining aliasing artifacts and noise while preserving the improved contrast. Lesion macro features were also faithfully reconstructed. Using early stage I results allowed for a swift training wall time of 30 hours—4 hours (20 epochs) for stage I and 26 hours (180 epochs) for stage II—on one Tesla P40 GPU.
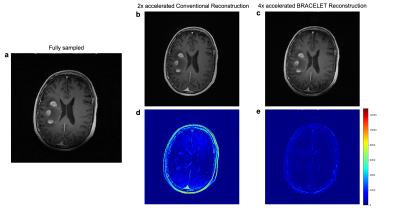
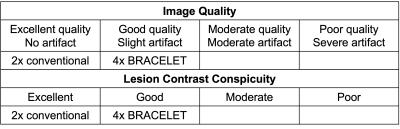
A neuroradiologist performed blind ratings of conventional 2-fold accelerated and 4-fold accelerated BRACELET images using an ordinal scale detailed in Table 1 on two measures: image quality and lesion contrast. 4-fold accelerated BRACELET results were rated good in both while the 2-fold accelerated conventional images were rated excellent in both. Figure 3 shows the difference images from the fully-sampled image. BRACELET results display superior lesion structural fidelity compared to the conventional imaging results.
Discussion
Utilizing a second stage in our workflow removed the dependence on one computationally complex neural network. It disentangled a multicoil, multi-channel problem into modular components, where more focus was assigned to a (significantly easier) single-channel reconstruction. FullConvMixer’s ability to concentrate on local structures aided rapid learning. BRACELET successfully removed major aliasing artifacts at higher accelerations and achieved comparable clinical scores. Although the conventional 2-fold accelerated images were rated higher, it should be noted that that workflow features significant post-processing. Finer anatomical structures (i.e., gray-white matter boundary, intra-tumorous) lag clinical reconstruction sharpness, possibly due to using L2-norm loss.Conclusion
This study investigated the feasibility of a modest DL workflow to reconstruct 4-fold accelerated 3D brain MRI datasets. The proposed BRACELET technique with 4-fold acceleration provides comparable image quality to the conventional images with 2-fold acceleration. Since T1-weighted images are acquired before and after contrast injection, the higher acceleration of BRACELET would remove the scan time of one of them, resulting in time savings of ~4-5 minutes in standard-of-care brain tumor MRI examinations.Acknowledgements
No acknowledgement found.References
- Hammernik, K., Klatzer, T., Kobler, E., Recht, M.P., Sodickson, D.K., Pock, T. and Knoll, F. (2018), Learning a variational network for reconstruction of accelerated MRI data. Magn. Reson. Med., 79: 3055-3071.
- Sriram, A., Zbontar, J., Murrell, T., Defazio, A., Zitnick, C. L., Yakubova, N., ... & Johnson, P. (2020, October). End-to-end variational networks for accelerated MRI reconstruction. In International Conference on Medical Image Computing and Computer-Assisted Intervention (pp. 64-73). Springer, Cham.
- Sriram, A., Zbontar, J., Murrell, T., Zitnick, C. L., Defazio, A., & Sodickson, D. K. (2020). GrappaNet: Combining parallel imaging with deep learning for multi-coil MRI reconstruction. In Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition (pp. 14315-14322).
- Schlemper, J., Caballero, J., Hajnal, J. V., Price, A. N., & Rueckert, D. (2017). A deep cascade of convolutional neural networks for dynamic MR image reconstruction. IEEE transactions on Medical Imaging, 37(2), 491-503.
- Knoll, F., Hammernik, K., Zhang, C., Moeller, S., Pock, T., Sodickson, D. K., & Akcakaya, M. (2020). Deep-learning methods for parallel magnetic resonance imaging reconstruction: A survey of the current approaches, trends, and issues. IEEE signal processing magazine, 37(1), 128-140.
- Zhang, T., Pauly, J.M., Vasanawala, S.S. and Lustig, M. (2013), Coil compression for accelerated imaging with Cartesian sampling. Magn Reson Med, 69: 571-582.
- Dosovitskiy, A., Beyer, L., Kolesnikov, A., Weissenborn, D., Zhai, X., Unterthiner, T., ... & Houlsby, N. (2020). An image is worth 16x16 words: Transformers for image recognition at scale. arXiv preprint arXiv:2010.11929.
- Tolstikhin, I., Houlsby, N., Kolesnikov, A., Beyer, L., Zhai, X., Unterthiner, T., ... & Dosovitskiy, A. (2021). Mlp-mixer: An all-mlp architecture for vision. arXiv preprint arXiv:2105.01601.
- Patches are all you need? (2021). Submitted to the tenth International Conference on Learning Representations. Under review.
- Zhu, B., Liu, J. Z., Cauley, S. F., Rosen, B. R., & Rosen, M. S. (2018). Image reconstruction by domain-transform manifold learning. Nature, 555(7697), 487-492.
Figures