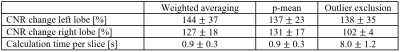
1154
Postprocessing Reduces Pulsation Artifacts and Increases Visibility of Liver Lesions in Flow-compensated Diffusion-weighted Imaging1University Hospital Erlangen, Erlangen, Germany, 2Klinikum Forchheim, Forchheim, Germany, 3Siemens Healthcare GmbH, Erlangen, Germany, 4Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Erlangen, Germany
Synopsis
Diffusion-weighted imaging of the liver is prone to the cardiac pulsation artifact, which can lead to reduced lesion visibility. We addressed this problem with a two-fold approach. First, flow-compensated diffusion weightings were used, which are known to reduce this artifact. Using a dataset of 40 patients suffering from focal liver lesions, we addressed the remaining signal voids with different postprocessing techniques, namely weighted averaging, the p-mean approach, and an outlier exclusion algorithm. The algorithms substantially increased the lesion visibility and further reduced the pulsation artifact. An evaluation of CNR and calculation time showed that weighted averaging was suited best.
Introduction
Diffusion-weighted imaging of the liver is prone to the cardiac pulsation artifact, in particular in the left liver lobe. This artifact manifests itself via signal voids in the affected region. As a consequence, lesions in the left liver lobe might be missed, which implies a lower diagnostical accuracy or even a wrong diagnosis. Two approaches that have been proposed to mitigate the severity of this artifact are the use of flow-compensated diffusion-weightings1-3 and the use of post-processing techniques that reduce the influence of affected image regions4-6. The aim of this work was to further reduce the severity of the pulsation artifact and to therewith increase the lesion visibility. To this end, a combination of the two approaches was used.Methods
Patient studyForty patients with multiple malignant focal liver lesions were scanned between January and August 2020. All exams were performed with informed consent and IRB approval. Images of 39 slices of the abdomen were acquired with a prototypical flow-compensated diffusion EPI sequence in free breathing. The b-values of 50 s/mm2 (1 average) and 800 s/mm2 (4 averages) were used in three orthogonal diffusion directions.
Postprocessing
All data processing was performed with Python 3.8. We focused on the examination of the b800 images because of the more dominant pulsation artifact compared to b50. Trace-weighted images were used as reference. These were computed by first averaging the four images per diffusion direction arithmetically and then averaging the three resulting images geometrically. We examined three other approaches:
1. p-mean approach according to Liau et al.4: For each diffusion direction, the new image was calculated voxel-wise by:
$$ y=\sqrt[p]{\frac{1}{4}\sum_{n=1}^{4}x_n^p} $$
Here, we used the factor p = 4. $$$x_n$$$ denotes the pixel values of the four images. Afterwards, the three diffusion images were averaged geometrically.
2. Weighted-averaging based on Ichikawa et al.5: For each diffusion direction, the new image was calculated voxel-wise using a weighted average. The weights of the images were calculated from blurred versions of the images (Gaussian filter, kernel size 11 x 11) in order to minimize the influence of single very bright or dark voxels:
$$ w_n=\frac{x_{n,\text{blurred}}^2}{\sum_{m=1}^4 x_{m,\text{blurred}}^2} $$
Here, $$$x_{n,\text{blurred}}$$$ denotes the voxel value of the blurred version of the n-th image. The new values were computed with the non-blurred images:
$$ y=\sum_{n=1}^{4}w_n x_n $$
where $$$x_n$$$ is the voxel value of the n-th un-blurred image. Afterwards, the three images were averaged geometrically.
3. Outlier exclusion algorithm inspired by ‘informed Restore’6: This approach makes use of the observation that the cardiac pulsation artifact induces signal dropout rather than signal increase.
First, a “badness map” was calculated: The standard deviation of the 12 images was calculated voxel-wise and divided by the reference image. This badness map was smoothed, also using a Gaussian filter (kernel size 21 x 21). Second, regions > 0.4 were marked as ‘bad’ regions, which means that they are probably corrupted by the pulsation artifact. In these regions, the lowest of the 12 signals was excluded for each voxel separately. This process was repeated iteratively, which means that in the next step, a new “badness map” is calculated using the remaining voxel values. In total, six iterations were allowed. If the voxel value had been the fourth one to be removed for a certain diffusion direction, then it was not removed in order to allow geometrical averaging.
Afterwards, the remaining values per diffusion direction were averaged arithmetically and the three resulting images were averaged geometrically.
Evaluation
To assess the lesion visibility, 18 representative lesions (nine in the left and right liver lobe each) of nine patients as well as corresponding ambient liver parenchyma were segmented. The CNR was calculated as
$$ CNR=\frac{S_\text{lesion}-S_\text{liver}}{\sigma_\text{liver}} $$
where $$$S_\text{lesion}$$$ and $$$S_\text{liver}$$$ denote the lesion and liver signal, respectively, and $$$\sigma_\text{liver}$$$ denotes the standard deviation of the liver tissue.
Results
The pulsation artifact can be substantially decreased (see Figure 1). Representative lesions are shown in Figure 2. The changes in the CNR relative to the reference images averaged over all segmented lesions in the respective lobe, and the averaged calculation time are shown in Table 1. All postprocessing techniques enhance the CNR. The increase in the left lobe is highest for weighted averaging, in the right lobe for the p-mean approach. The smallest change occurs for the outlier exclusion algorithm in the right lobe, which is also the slowest algorithm.Discussion
The three examined postprocessing algorithms are all suitable to increase lesion visibility. According to the evaluation of the CNR and the calculation time, weighted averaging is the best approach to increase lesion visibility. The p-mean approach might be better in the right lobe, but as the pulsation artifact occurs predominantly in the left liver lobe, this only plays a minor role. The small CNR change in the right lobe in the outlier exclusion algorithm is due to its restriction on regions with high relative standard deviation, which are predominantly in the left lobe. The outlier exclusion algorithm is relatively slow because of its iterative architecture.Conclusion
The proposed weighted averaging is a well-suited and fast algorithm to increase lesion visibility and reduce the pulsation artifact in the left liver lobe.Acknowledgements
No acknowledgement found.References
1. Rauh, S.S., et al., A mixed waveform protocol for reduction of the cardiac motion artifact in black-blood diffusion-weighted imaging of the liver. Magn Reson Imaging, 2020. 67: p. 59-68.
2. Ozaki, M., et al., Motion artifact reduction of diffusion-weighted MRI of the liver: use of velocity-compensated diffusion gradients combined with tetrahedral gradients. J Magn Reson Imaging, 2013. 37(1): p. 172-8.
3. Aliotta, E., H.H. Wu, and D.B. Ennis, Convex optimized diffusion encoding (CODE) gradient waveforms for minimum echo time and bulk motion-compensated diffusion-weighted MRI. Magn Reson Med, 2017. 77(2): p. 717-729.
4. Liau, J., et al., Cardiac motion in diffusion-weighted MRI of the liver: artifact and a method of correction. J Magn Reson Imaging, 2012. 35(2): p. 318-27.
5. Ichikawa, S., et al., Improving the Quality of Diffusion-weighted Imaging of the Left Hepatic Lobe Using Weighted Averaging of Signals from Multiple Excitations. Magn Reson Med Sci, 2019. 18(3): p. 225-232.
6. Chang, L.C., L. Walker, and C. Pierpaoli, Informed RESTORE: A method for robust estimation of diffusion tensor from low redundancy datasets in the presence of physiological noise artifacts. Magn Reson Med, 2012. 68(5): p. 1654-63.
Figures
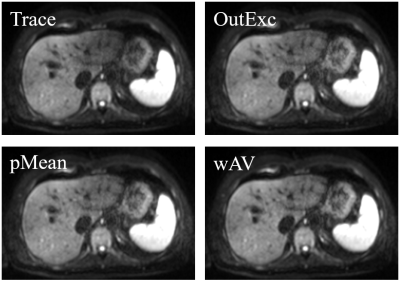
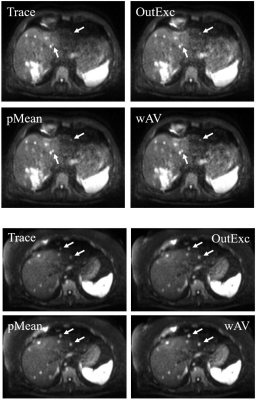
Figure 2: Two examples for different visibilities of lesions. The trace-weighted image (Trace) is regarded as reference image. The lesions marked by the arrows are better visible in the images processed with the outlier exclusion algorithm (OutExc), the p-mean approach (pMean) and the weighted averaging (wAV).