1148
Diffusion-weighted MEGA-PRESS spectroscopy1Department of Chemistry, University of Bergen, Bergen, Norway
Synopsis
The editing capabilities of the MEGA-PRESS sequence are combined with diffusion-weighing to enable both quantification and diffusion measurements of less abundant brain metabolites. The technique, labelled DW-MEGA-PRESS, was tested for combined editing and diffusion-weighting of GABA. Obtained data give reliable results for GABA concentrations and corresponding diffusion coefficients from in vitro experiments. When applied to in vivo acquisitions, reliable diffusion coefficients for the dominating metabolites were obtained. GABA could be reliably quantified, but the signal-to-noise ratio was not sufficient for a reliable determination of its diffusion coefficient. This could be improved by further optimization of scanning and post-processing protocols.
Intro
Spectral editing in 1H-MRS enables quantification of brain metabolite signals that are unobtainable using conventional MRS techniques, such as PRESS or STEAM. MEGA-PRESS1 utilizes highly selective rf-pulses to refocus specific J-coupled spins in a molecule. Applying these selective rf-pulses in an ‘ON’ and ‘OFF’ scheme results in an edited spectrum that reveals signals from less abundant metabolites, which are otherwise obscured due to strong overlap with more dominating signals. The diffusion behavior of specific signals can be investigated by adding diffusion-weighting gradients to PRESS or STEAM2. Diffusion-weighted MRS (DW-MRS) allows for extraction of cellular information because the apparent diffusion coefficient (ADC) depends on the local geometry2. However, DW-MRS has so far been challenging in the measuring of ADC’s of less abundant brain metabolites, such as γ-aminobutyric acid (GABA)3. We propose to combine the editing capabilities of the MEGA-PRESS sequence with diffusion-weighing gradients to enable measurement of the ADC of less abundant metabolites. We have labelled this technique DW-MEGA-PRESS. The technique was tested for combined editing and diffusion-weighting of GABA both in vitro and in vivo.Method
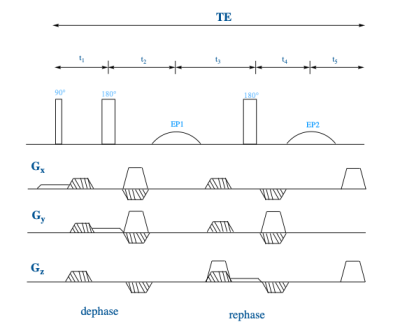
The diffusion block was included in the MEGA-PRESS sequence by superimposing bipolar diffusion-weighting gradients on the already existing spoiler gradients (Fig. 1). This keeps the echo time within the limits specified by the strength of the J-coupling in the editing molecule and allows for easier access to stronger diffusion-weighing when the gradient strength is a limitation. In vitro experiments were performed at 25 °C on a 11.7 T Bruker 500 WB spectrometer using a series of phosphate-buffer solutions with GABA concentrations varying in the range of 2-10 mM and on a liquid phantom (choline (Cho, 3.8 mM), creatine (Cr, 12.0 mM), lactate (Lac, 5.6 mM), myoinositole (mI, 8.4 mM), glutamic acid (Glu, 16.0 mM) and N-acetylactic acid (NAA, 12.0 mM), Glutathione (GSH, 2.7 mM) and (GABA, 9.8 mM)). ‘ON’ and ‘OFF’ spectra were acquired using DW-MEGA-PRESS with 14 b-values in the range 0 - 4.22× 109 s/m2, 256 averages, TE = 68, TR = 5000, and a 4 x 4 x 4 mm voxel. In vivo spectra were acquired at 37 °C using a 7 T Bruker Pharmascan with a 4-channel head coil. Rats (n=8) were sedated using isoflurane. A 4 x 4 x 4 mm voxel was placed in the cerebral cortex guided by a T1-MPRAGE acquisition. Using DW-MEGA-PRESS a series of ‘ON’ and ‘OFF’ spectra were recorded with 5 b-values in the range 0 – 4.56 × 109 s/m2, 32 averages, TE = 68, and TR = 2500. The total experiment time per animal was 40-50 minutes. All post-processing was done using an in-house Matlab script. The GABA/NAA ratio was used for quantification of GABA.Results
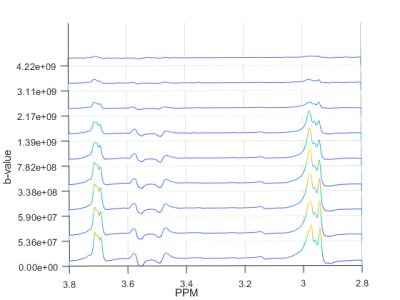
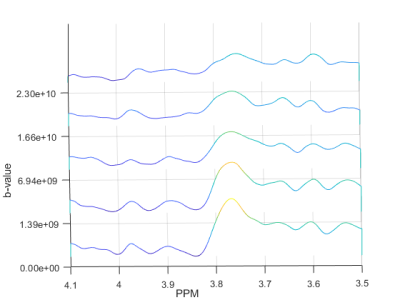
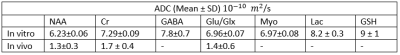
The in vitro data from DW-MEGA-PRESS at b = 0 (regular MEGA-PRESS) showed a good correlation (R2 = 0.998) between the intensity of the edited signal at 3.0 ppm and the concentrations of GABA. Diffusion-weighted edited spectra from the phantom solution are shown in Fig. 2. The values (Table 1) are in good correspondence with corresponding data obtained using diffusion-ordered NMR-spectroscopy performed in our lab and by Landheer et. al3. The GABA/NAA ratios in the in vivo experiments (b = 0) were determined to be 0.21 ± 0.03 (n = 8). Using DW-‘OFF’ spectra, ADC’s of the most abundant metabolites could be determined. The DW-weighted edited (‘ON’-‘OFF’) spectra did not have sufficient quality to determine the ADC of GABA. However, using the co-edited signal at 3.75 ppm the ADC of Glx could be determined with a satisfactory accuracy. Diffusion-weighted edited spectra of the Glx-signal are shown in Fig. 3. The ADC values obtained in vivo are found in Figure 4.Discussion
Spectral editing of GABA using data acquired using the proposed DW-MEGA-PRESS sequence in phantom solutions verifies that the method can obtain good quality estimates of GABA concentrations. Furthermore, diffusion-weighted data for all the metabolites, including the data for GABA obtained from edited spectra, results in ADC values of high reliability. The in vivo data results in reproducible values for GABA/Cr ratios, and that correspond well with values previously obtained in rats using MEGA-PRESS4. Unfortunately, the signal-to-noise ratio of the edited DW-weighted signal from GABA was not sufficient to enable a reliable estimate of its ADC. This could be improved by increasing the number of averages, or by an improved and optimized pipeline for post-processing5. However, the co-edited DW-weighted signal from Glx had sufficient stability to enable a reliable ADC estimation, showing the in vivo potential of the DW-MEGA-PRESS sequence for determination of ADC’s from signals having sufficient signal-to-noise ratios.Conclusion
Using DW-MEGA-PRESS it is possible to achieve simultaneous spectral editing and diffusion weighing, potentially enabling DW-studies of less abundant brain metabolites. However, preliminary results from in vivo experiments show that it can be challenging to acquire DW-data with sufficient quality for the less abundant metabolites, and further development and optimization of scanning protocols should be performed.Acknowledgements
Thank you to the Animal Department at Haukeland University Hospital for assisting in the in vivo experiments with animal handling and access to the facilities.References
1. Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998 Oct;11(6):266-72.
2. Marco Palombo, Noam Shemesh et. al. Insights into brain microstructure from in vivo DW-MRS. NeuroImage. Volume 182. 2018. Pages 97-116, ISSN 1053-8119, https://doi.org/10.1016/j.neuroimage.2017.11.028.
3. Landheer, K., Schulte, R. et. al. (2017), Diffusion-weighted J-resolved spectroscopy. Magn. Reson. Med., 78: 1235-1245. https://doi.org/10.1002/mrm.26514
4. Sawiak, S.J., Jupp, B. et. al. (2016), In vivo γ-aminobutyric acid measurement in rats with spectral editing at 4.7T. J. Magn. Reson. Imaging, 43: 1308-1312. https://doi.org/10.1002/jmri.25093
5.
Guo, J,
Gang, Z, et. al. In vivo detection and automatic analysis of GABA in the mouse brain with MEGA-PRESS at 9.4 T. NMR in Biomedicine. 2018; 31:e3837. https://doi.org/10.1002/nbm.3837
Figures