1146
Multi-Shot Liver Diffusion MRI Using Variable Auto-Calibrating (vARC) Sampling Across Averages1GE Healthcare, Bangalore, India, 2Indian Institute of Technology Madras, Chennai, India
Synopsis
Obesity is a key biomarker of liver pathology. Diffusion MRI in liver is key protocol in the absence on intravenous contrast agent and also provides additional insights on diffuse liver diseases and lesions However, its usage is limited by the inability to use multi-channel surface coil for obese liver patients in non-wide-bore MRI scanners. A variable k-space sampling scheme and auto-calibrating image reconstruction technique, vARC, is proposed in this abstract to reduce the amount of distortion and improve the image quality of Liver DWI acquired with single channel volume coil.
PURPOSE
Acquire Liver diffusion with good SNR and minimal distortion in large patients for whom multi-channel surface coil acquisition is not possible due to narrow space between the magnet bore and abdomen.INTRODUCTION
Diffusion MRI in liver is a key protocol in the absence on intravenous contrast agent and also provides additional insights on diffuse liver diseases and lesions [1,2,3,4]. Liver is surrounded by multiple interfaces with big susceptibility changes such as lung-liver interface which accentuate the distortion present in the diffusion MRI acquired using EPI data acquisition [4,5]. These distortions in the phase encoding direction are directly proportional to the effective echo-spacing between the neighboring phase encode lines [3]. Liver has small T2 value and presence of magnetic field inhomogeneity over liver leads to rapid decay of the diffusion encoded signal in liver EPI-DWI [5]. Multi-channel coil acquisition with parallel imaging [7] and/or multi-shot [8] imaging techniques reduces the effective echo spacing and the total number of the phase encoding lines in EPI readout is essential for liver Diffusion MRI [2].Obesity is the key marker of the liver disease [6] where the usage of the multi-channel surface coil may not be possible due to the narrow space between the magnet bore and abdomen. Thereby, limiting the adoption of liver diffusion MRI in clinical practice [3]. In this abstract we have proposed a variable auto-calibrating (vARC) k-space sampling scheme and image reconstruction algorithm to reduce the effective echo-spacing and total EPI readout duration for single channel coil acquisition.
METHODS
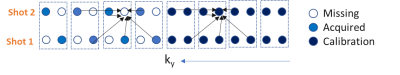
The proposed vARC technique composed of novel k-space sampling scheme and image reconstruction algorithm.k-space sampling: DWI is typically acquired with multiple number of excitations/averaging (NEX). Central k-space contributes to SNR while outer k-space locations contribute to the edges in the image. The reduction in echo spacing with total EPI-readout time reduction is done while maintaining the SNR by extending the sampling pattern of the fast k-t MRI techniques [9,10,11] k-NEX for EPI-DWI. The central k-space is fully sampled, and outer k-space is subsampled by an acceleration factor R. The subsampling of outer k-space is shifted over the signal averages so that all the outer k-space lines are acquired once over R signal averages. Figure 1 shows the sampling scheme for R=2.
Image Reconstruction: The microscopic motion during the diffusion encoding adds significant amount of phase in the DWI of each signal average [5]. As shown in Figure 1, the missing k-space locations are estimated for each signal average using the linear combination of the neighboring k-space locations for all the signal averages [10,11]. Weights of the linear combination is estimated/self-calibrated using the central fully sampled k-space.
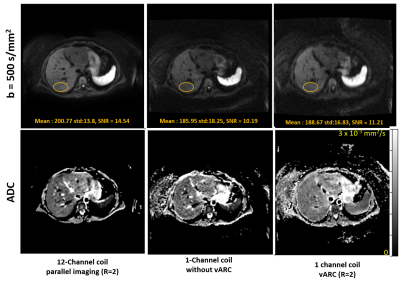
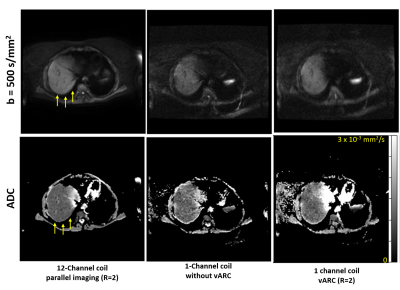
Volunteer Scanning: A healthy subject was scanned using the IRB approved study with informed consent on a commercial 1.5T MRI scanner for liver using 12 channel anterior array coil and single channel volume coil. The EPI-DWI pulse sequence was updated to incorporate the proposed k-space sampling scheme. Axial DWI image with b=50 and 500 s/mm2 was acquired using the 12 channel surface coil with parallel imaging acceleration of 2 (12Ch-PI2) and using the single channel volume coil with vARC with acceleration factor of 2 (1Ch-vARC2) and without proposed vARC sampling pattern (1Ch-No-vARC). The scan time for three DWI scans were similar.
RESULTS AND DISCUSSION
Figure 2 and 3 shows DWI image for b=500 s/mm2 and estimated ADC maps. The 12Ch-PI2 images have parallel image acceleration of 2 to reduce the echo spacing and has the least distortion. The 1Ch-No-vARC images have longer EPI readout and have double the echo spacing compared to the 12Ch-PI2 images leading to poor SNR and doubled the distortions. The 1Ch-vARC2 images have better SNR compared to 1Ch-No-vARC images and have distortions that is better than 1Ch-No-vARC images and poor than 12Ch-PI2 images. This is because the central k-space of 1Ch-vARC2 has echo-spacing as that 1Ch-No-vARC images and outer k-space has echo-spacing as that of 12Ch-PI2. Therefore, edges of 1Ch-vARC2 from outer k-space is less distorted compared to the contrast of 1Ch-vARC2 from central k-space.CONCLUSION
Less-distorted high-quality liver DWI with single channel coil acquisition is key to enable widespread adoption of DWI in liver MRI due to the high prevalence of liver disease in obese patients who may not be scanned with surface coil especially in the non-wide bore commercial MRI scanners. In this abstract Variable Auto-Calibrating (vARC) technique is proposed for single channel DWI with multiple signal averages. Initial results demonstrated improved SNR and reduced distortion in liver DWI, however, further study is warranted.Acknowledgements
No acknowledgement found.References
[1] Kele, P.G. and van der Jagt, E.J., 2010. Diffusion weighted imaging in the liver. World journal of gastroenterology: WJG, 16(13), p.1567.
[2] Shenoy-Bhangle, A., Baliyan, V., Kordbacheh, H., Guimaraes, A.R. and Kambadakone, A., 2017. Diffusion weighted magnetic resonance imaging of liver: Principles, clinical applications and recent updates. World journal of hepatology, 9(26), p.1081.
[3] Donato, H., França, M., Candelária, I. and Caseiro-Alves, F., 2017. Liver MRI: from basic protocol to advanced techniques. European journal of radiology, 93, pp.30-39.
[4] Taouli, B. and Koh, D.M., 2010. Diffusion-weighted MR imaging of the liver. Radiology, 254(1), pp.47-66.
[5] Le Bihan, D., Poupon, C., Amadon, A. and Lethimonnier, F., 2006. Artifacts and pitfalls in diffusion MRI. Journal of Magnetic Resonance Imaging: An Official Journal of the International Society for Magnetic Resonance in Medicine, 24(3), pp.478-488.
[6] Marchesini, G., Moscatiello, S., Di Domizio, S. and Forlani, G., 2008. Obesity-associated liver disease. The Journal of Clinical Endocrinology & Metabolism, 93 (11_supplement_1), pp.s74-s80.
[7] Brau, A.C., Beatty, P.J., Skare, S. and Bammer, R., 2008. Comparison of reconstruction accuracy and efficiency among autocalibrating data‐driven parallel imaging methods. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine, 59(2), pp.382-395.
[8] Chen, N.K., Guidon, A., Chang, H.C. and Song, A.W., 2013. A robust multi-shot scan strategy for high-resolution diffusion weighted MRI enabled by multiplexed sensitivity-encoding (MUSE). Neuroimage, 72, pp.41-47.
[9] Tsao, J., 2002. On the UNFOLD method. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine, 47(1), pp.202-207.
[10] Huang, F., Akao, J., Vijayakumar, S., Duensing, G.R. and Limkeman, M., 2005. k‐t GRAPPA: A k‐space implementation for dynamic MRI with high reduction factor. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine, 54(5), pp.1172-1184.
[11] Lai, P., Fung, M.M., Vasanawala, S.S. and Brau, A.C., 2012. Single breathhold three-dimensional cardiac cine MRI with whole ventricular coverage and retrospective cardiac gating using kat ARC. Journal of Cardiovascular Magnetic Resonance, 14(1), pp.1-2.
Figures