1143
Quantitative measurements of GABA in mouse brain using MR Spectroscopy at 7T
Mohamed Tachrount1, Jason Lerch1,2, and Stuart Clare1
1Wellcome Centre for Integrative Neuroimaging (FMRIB), Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, United Kingdom, 2Mouse Imaging Centre (MICe), University of Toronto, Toronto, ON, Canada
1Wellcome Centre for Integrative Neuroimaging (FMRIB), Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, United Kingdom, 2Mouse Imaging Centre (MICe), University of Toronto, Toronto, ON, Canada
Synopsis
The goals of this study were to implement a MEGA-sLASER sequence with prospective field drift correction on a preclinical scanner, develop the processing tools and validate them on a group of wild type mice.
Introduction
γ-Aminobutyric acid (GABA) is the primary inhibitory neurotransmitter in the mammalian brain. It plays a major role in the function of the healthy brain and in a variety of neurological and psychiatric disorders including epilepsy [1], autism [2], schizophrenia [3]. To quantify its concentration in vivo, 1H Magnetic Resonance spectral editing is widely used in human studies [4]. However, there is a very limited number of studies applying those techniques to the GABA quantification in animal models [5,6,7] despite their significant role in understanding the normal brain function and the pathophysiology of several diseases. It could be due to several factors including the low GABA concentration and the size of the brain, the respiratory motion, the main magnetic field drift, large chemical shift displacement errors, and the lack of standard pulse sequences on preclinical scanners. The goals of this study were to implement a MEGA-sLASER sequence [8] with prospective field drift correction on a preclinical scanner, develop the processing tools and validate them on a group of wild type mice.Materials and methods
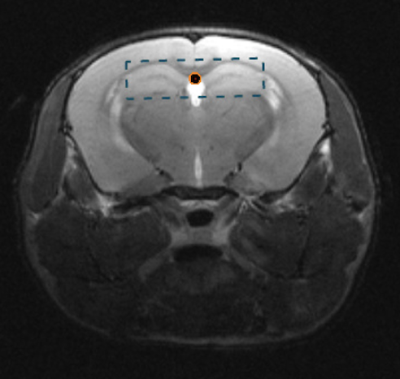
Animal preparation: Experiments were performed following the UK Home Office guidelines and were approved by the local animal care and use committee. Eight healthy adult male C57BL/6 mice (30±3g) were scanned using a 7T Bruker scanner and Bruker 86mm volume/cryoprobe surface coils to transmit/receive the signal. Animals were anesthetized using isoflurane mixed with air. The concentration of isoflurane was adjusted to maintain the respiration rate in the range of 80–90 breaths/min. High resolution T2 images acquired using RARE sequence were used for the positioning of the MRS ROIs (Fig.1). MR protocol: 1H MR spectra were obtained from voxels located within the thalamus (5x2x2mm3) and hippocampus (5.2x2x2mm3) using single voxel MEGA-sLASER sequence. The water and the outer volume signals were saturated using a combination of VAPOR and three OVS modules [9]. The acquisition parameters were: TE/TR=68ms/3000ms, 2048 samples, BW=2950 Hz, NA=8, NR=15, and TA=12min. The editing was performed using Gaussian RF pulses (BW=150Hz) applied at 1.9ppm (ON) and 7.5ppm (OFF). To correct for the field drift during the scan, navigators (non-selective excitation, FA=1deg, 128 samples) were interleaved with the MRS data acquisition. The spectra were processed using Matlab script and a modified version of Gannet [www.gabamrs.com, 10] to load the raw data, align the transients, subtract the ON from the OFF spectra, and quantify the GABA.Results
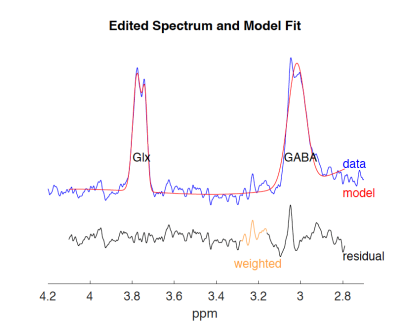
Fig. 2 depicts typical spectra obtained from thalamus and hippocampus using our sequence. The SNR of the hippocampus spectra was higher than thalamus given the distance from the surface coil. The coefficients of variation (CV) within the thalamus and hippocampus were 11.3% and 18.6%, respectively.Discussion and conclusion
To the best of our knowledge, this study demonstrated for the first time the feasibility of the application of MEGA-sLASER to the quantification of GABA in mice brain. The effects of the chemical shift displacements errors should be less pronounced compared to MEGA-PRESS [5]. With the interleaved navigator and prospective frequency offset correction, no substantial drift of water and metabolites frequencies were observed.Acknowledgements
This work was supported by the Wellcome Trust (grant 202788/Z/16/Z). The Wellcome Centre for Integrative Neuroimaging is supported by core funding from the Wellcome Trust (203139/Z/16/Z).References
References: 1- D M Treiman. GABAergic mechanisms in epilepsy. Epilepsia. 2001;42 Suppl 3:8-12. 2- J Horder, M M. Petrinovic, M A. Mendez, a Bruns, T Takumi, W Spooren, G J. Barker, B Künnecke, D G. Murphy. Glutamate and GABA in autism spectrum disorder - a translational magnetic resonance spectroscopy study in man and rodent models. Translational Psychiatry volume 8, Article number: 106 (2018) 3- A. Egerton, G Modinos, D Ferrera, P McGuire Neuroimaging studies of GABA in schizophrenia: a systematic review with meta-analysis. Translational Psychiatry volume 7, page e1147 (2017) 4- R. Rideaux, M. Mikkelsen, R.A.E. Edden. Comparison of methods for spectral alignment and signal modelling of GABA-edited MR spectroscopy data. NeuroImage Volume 232, 117900 5- Jia Guo, Zhu Gang, Yanping Sun, Andrew Laine, Scott A. Small, Douglas L. Rothman. In vivo detection and automatic analysis of GABA in the mouse brain with MEGA-PRESS at 9.4 T. Volume31, Issue1, January 2018, e3837 6- W. Saskia van der Hel, Pieter van Eijsden, Ineke W. M. Bos, Robin A. de Graaf, Kevin L. Behar, Onno van Nieuwenhuizen, Pierre N. E. de Graan, Kees P. J. Braun. In vivo MRS and histochemistry of status epilepticus-induced hippocampal pathology in a juvenile model of temporal lobe epilepsy. Volume26, Issue2, February 2013, Pages 132-140 7- Sophie Hamelin, Vasile Stupar, Lucile Mazière, Jia Guo, Wafae Labriji, Chen Liu, Ludiwine Bretagnolle, Sandrine Parrot, Emmanuel L Barbier, Antoine Depaulis, Florence Fauvelle. In vivo γ-aminobutyric acid increase as a biomarker of the epileptogenic zone: An unbiased metabolomics approach. Epilepsia. 2021 Jan;62(1):163-175. 8- Anna Andreychenko, Vincent O. Boer, Catalina S. Arteaga de Castro, Peter R. Luijten, Dennis W. J. Klomp. Efficient spectral editing at 7 T: GABA detection with MEGA-sLASER. Magnetic Resonance in Medicine. Volume 68, Issue 4 p. 1018-1025 9- Tkác I, Starcuk Z, Choi IY, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med. 1999 Apr; 41(4):649-56. Ashley D. Harris, Muhammad G. Saleh, and Richard A.E. Edden. Edited 1H Magnetic Resonance Spectroscopy In Vivo: Methods and Metabolites. Magn Reson Med. 2017 Apr; 77(4): 1377–1389
DOI: https://doi.org/10.58530/2022/1143