1139
Skeletal muscle energetics and function in sickle cell disease murine model: a synergetic effect of hydroxyurea and endurance training?1Aix-Marseille Univ, CNRS, CRMBM, Marseille, France, 2Laboratoire Interuniversitaire de Biologie de la Motricité, Chambéry, France
Synopsis
In sickle cell disease (SCD), hydroxyurea can increase foetal haemoglobin production and restore oxygen-carrying capacity of patients. As endurance training is also known to improve muscle aerobic capacity, one can wonder whether hydroxyurea and endurance training can have a synergetic effect. Townes mice (model of SCD) have been trained with and without hydroxyurea for 8 weeks. Muscle energetics and function were assessed in response to a rest-stimulation-recovery protocol before and after interventions. While training increased force production, mitochondrial function was improved when training was combined to hydroxyurea supplementation. In SCD, this combination would have a synergetic effect in muscle energetics.
Introduction
Sickle cell disease (SCD) is an inherited hemoglobinopathy resulting in a production of sickle Hb (HbS) which can polymerize in deoxygenated conditions, eventually leading to the sickling of red blood cells (RBC)1. SCD patients suffer from severe chronic haemolytic anaemia2 and arterial desaturation3,4, resulting in tissue hypoxia5,6. All organs ache from these alterations, including skeletal muscle7–9. The decreased oxygen-carrying capacity can alter energetics and function in response to exercise in patients and mice model. Any strategy which could reverse metabolic and functional defects could be of interest.Hydroxyurea (HU) is commonly used is SCD in order to produce foetal haemoglobin (HbF)10,11, reduce sickle haemoglobin level and ultimately alleviate SCD-related symptoms12. HU treatment has also been related to an increase in anaerobic and aerobic capacities in patients13.
Regular physical activity can also be considered as an interesting therapeutic strategy. Endurance training has already been proven as efficient for improving oxygen supply14,15. In SCD patients, moderate-intensity endurance-exercise has been reported safe in adults and linked to an improved functional capacity16. These results have been further confirmed in an SCD animal model17.
Overall, one can wonder whether endurance training and hydroxyurea supplementation can have a synergetic effect on muscle function and more specifically on muscle energetics considering the tight link between energetics and oxygenation18.
Methods
Townes mice (the SCD murine model) are expressing the human haemoglobin and display the typical clinical phenotype of SCD patients such as anaemia, sickling and organs dysfunction19. Skeletal muscle energetics and function of twenty-one mice (2 ± 0.5 months-olds) were assessed in response to a standardized Rest-Stimulation(1Hz)-Recovery protocol before and after interventions. Investigations were conducted using an homebuilt experimental setup that has been designed to be operational inside the PharmaScan® gradient insert (Bruker BioSpin MRI Gmbh, Ettlinge, Germany)20. The setup allows (i) magnetic resonance imaging (MRI), (ii) muscle force measurements and (iii) 31P-magnetic resonance spectroscopy (31P-MRS). MRI was used to get anatomical information about the hindlimb. The posterior hindlimb muscle mechanical performance was assessed with a dedicated homemade ergometer consisting of a foot pedal coupled to a force transducer. 31P-MRS was used to dynamically monitor the levels of high-energy phosphorylated compounds and acidosis.Nine mice (END) were subjected to an 8-weeks endurance training on a treadmill, with 55 minutes sessions five days a week, including one-week acclimatation17. Training started at 15m/min and increased progressively during the weeks up to 17m/min. Twelve mice (END-HU) were submitted to an 8-weeks hydroxyurea supplementation in drinking water at 50 mg/kg/day21, associated with endurance training. Assessments conducted before intervention are indicated through number 1 (END1) and post-intervention session with number 2 (END2).
Results
As illustrated in figure 1, total specific peak force was significantly higher in the post-intervention groups (END2 and END-HU2, +46% and +39% respectively, p < 0.05) as compared to the corresponding pre-intervention conditions (END1 and END-HU1), with no other significant difference between groups.The rate of PCr recovery (VPCrrec) was accelerated in END-HU2 as compared to END-HU1 (+83%, figure 2, p < 0.05). No other significant difference was found after endurance intervention only.
Discussion
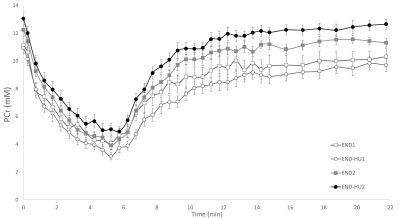
Both type of interventions allowed to increase total specific peak force as compared to their pre-intervention condition. It has been previously reported that HU enhanced anaerobic and aerobic capacity in patients13 and that endurance training also increased skeletal muscle function16. The present result further supports the beneficial effects of each and both interventions. However, a synergetic effect was not identified.Of interest, the rate of PCr recovery, a well-known index of mitochondrial function22, was accelerated as a result of the combination between HU and endurance training whereas the corresponding kinetics was not modified by the endurance training modality. One must keep in mind that PCr recovery kinetics is tightly linked to oxygenation. Indeed, in hyperoxic conditions, PCr recovery kinetics can be accelerated18, whereas hypoxia led to a reduced rate of PCr resynthesis22. Considering the anaemic status of Townes mice, one could have expected a reduced VPCrrec19. Recent studies reported an unchanged recovery kinetics in women with SCD23 and Townes mice24 compared to their control counterparts thereby illustrating that the mitochondrial function might not be compromised in SCD.
The synergetic effect of both interventions can be compared to the effects of hyperoxia in a trained population. It has been reported that trained subject can benefit from hyperoxia given their larger mitochondrial content. More specifically, an hyperoxia-mediated accelerated PCr recovery has been measured in trained subjects whereas it was not in sedentary controls22. Endurance training would lead to an increased mitochondrial content25,26, so that the beneficial effect in hyperoxia could be observed. Such an effect would not be observed when mitochondrial content is unchanged. In our case, HU might create the hyperoxic condition given the increased oxygen delivery to skeletal muscle related to the increased production of HbF27 and NO10. Training only would not be effective in Townes mice likely because of the anaemic status.
Overall, the combination of endurance training and hydroxyurea supplementation did improve the PCr recovery kinetics of Townes mice.
Conclusion
In conclusion, a potential synergetic effect of endurance training and hydroxyurea treatment has been demonstrated for skeletal muscle energetic and function in a sickle cell disease mice model.Acknowledgements
This work was partly funded by France Life Imaging (grant ANR-11-INBS-0006), AFM and Region Sud.
Hydroxyurea has been kindly provided by the ADDMEDICA company.
Constance MICHEL is a PhD student supported by Aix-Marseille University.
CRMBM is a research lab funded by CNRS.
Laboratoire Inter Universitaire de Biologie de la Motricité is a research group funded by the Université Savoie Mont-Blanc.
References
1. Vekilov, P. G. Sickle-cell haemoglobin polymerization: is it the primary pathogenic event of sickle-cell anaemia? Br J Haematol 139, 173–184 (2007).
2. Rees, D. C., Williams, T. N. & Gladwin, M. T. Sickle-cell disease. The Lancet 376, 2018–2031 (2010).
3. Quinn, C. T. & Sargent, J. W. Daytime steady-state haemoglobin desaturation is a risk factor for overt stroke in children with sickle cell anaemia. Br J Haematol 140, 336–339 (2008).
4. Setty, B. N. Y., Stuart, M. J., Dampier, C., Brodecki, D. & Allen, J. L. Hypoxaemia in sickle cell disease: biomarker modulation and relevance to pathophysiology. The Lancet 362, 1450–1455 (2003).
5. Nahavandi, M., Tavakkoli, F., Hasan, S. P., Wyche, M. Q. & Castro, O. Cerebral oximetry in patients with sickle cell disease. Eur J Clin Invest 34, 143–148 (2004).
6. Nahavandi, M., Nichols, J. P., Hassan, M., Gandjbakhche, A. & Kato, G. J. Near-infrared spectra absorbance of blood from sickle cell patients and normal individuals. Hematology 14, 46–48 (2009).
7. Waltz, X. et al. Normal Muscle Oxygen Consumption and Fatigability in Sickle Cell Patients Despite Reduced Microvascular Oxygenation and Hemorheological Abnormalities. PLoS ONE 7, e52471 (2012).
8. Ravelojaona, M. et al. Evidence for a Profound Remodeling of Skeletal Muscle and Its Microvasculature in Sickle Cell Anemia. The American Journal of Pathology 185, 1448–1456 (2015).
9. Charlot, K. et al. Cerebral and muscle microvascular oxygenation in children with sickle cell disease: Influence of hematology, hemorheology and vasomotion. Blood Cells, Molecules, and Diseases 65, 23–28 (2017).
10. Cokic, V. P. et al. Hydroxyurea induces fetal hemoglobin by the nitric oxide–dependent activation of soluble guanylyl cyclase. J. Clin. Invest. 111, 231–239 (2003).
11. Almeida, C. B. et al. Hydroxyurea and a cGMP-amplifying agent have immediate benefits on acute vaso-occlusive events in sickle cell disease mice. Blood 120, 2879–2888 (2012).
12. Charache, S. et al. Effect of Hydroxyurea on the Frequency of Painful Crises in Sickle Cell Anemia. N Engl J Med 332, 1317–1322 (1995).
13. Hackney, A. C. et al. Effects of Hydroxyurea Administration on the Body Weight, Body Composition and Exercise Performance of Patients with Sickle-Cell Anaemia. Clinical Science 92, 481–486 (1997).
14. Mairbäurl, H. Red blood cells in sports: effects of exercise and training on oxygen supply by red blood cells. Front. Physiol. 4, (2013).
15. Nader, E. et al. Blood Rheology: Key Parameters, Impact on Blood Flow, Role in Sickle Cell Disease and Effects of Exercise. Front. Physiol. 10, 1329 (2019).
16. Gellen, B. et al. Moderate-intensity endurance-exercise training in patients with sickle-cell disease without severe chronic complications (EXDRE): an open-label randomised controlled trial. The Lancet Haematology 5, e554–e562 (2018).
17. Chatel, B. et al. Endurance training reduces exercise-induced acidosis and improves muscle function in a mouse model of sickle cell disease. Molecular Genetics and Metabolism 123, 400–410 (2018).
18. Haseler, L. J., Hogan, M. C. & Richardson, R. S. Skeletal muscle phosphocreatine recovery in exercise-trained humans is dependent on O 2 availability. Journal of Applied Physiology 86, 2013–2018 (1999).
19. Wu, L.-C. et al. Correction of sickle cell disease by homologous recombination in embryonic stem cells. Blood 108, 1183–1188 (2006).
20. Giannesini, B. et al. A strictly noninvasive MR setup dedicated to longitudinal studies of mechanical performance, bioenergetics, anatomy, and muscle recruitment in contracting mouse skeletal muscle. Magn. Reson. Med. 64, 262–270 (2010).
21. Lebensburger, J. D., Pestina, T. I., Ware, R. E., Boyd, K. L. & Persons, D. A. Hydroxyurea therapy requires HbF induction for clinical benefit in a sickle cell mouse model. Haematologica 95, 1599–1603 (2010).
22. Haseler, L. J., Lin, A. P. & Richardson, R. S. Skeletal muscle oxidative metabolism in sedentary humans: 31 P-MRS assessment of O 2 supply and demand limitations. Journal of Applied Physiology 97, 1077–1081 (2004).
23. Chatel, B. et al. In vivo muscle function and energetics in women with sickle cell anemia or trait: a 31 P-magnetic resonance spectroscopy study. Journal of Applied Physiology 130, 737–745 (2021).
24. Chatel, B. et al. Moderate and intense muscular exercises induce marked intramyocellular metabolic acidosis in sickle cell disease mice. Journal of Applied Physiology 122, 1362–1369 (2017).
25. Holloszy, J. O. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem 242, 2278–2282 (1967).
26. Holloszy, J. O. & Booth, F. W. Biochemical Adaptations to Endurance Exercise in Muscle. Annu. Rev. Physiol. 38, 273–291 (1976).
27. Platt, O. et al. Hydroxyurea enhances fetal hemoglobin production in sickle cell anemia. The Journal of Clinical Investigation 74, 652–6 (1984).
Figures
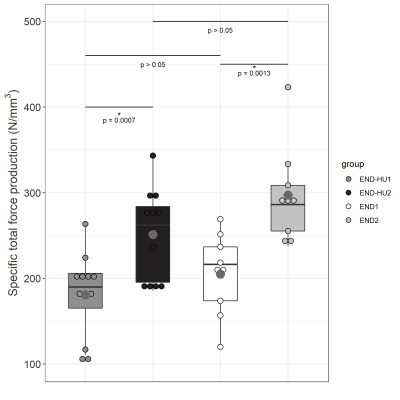
Specific total force production from the 6 minutes moderate exercise of each condition before and after intervention. Results are presented in boxplot. Statistical results are assessed on the boxplot.
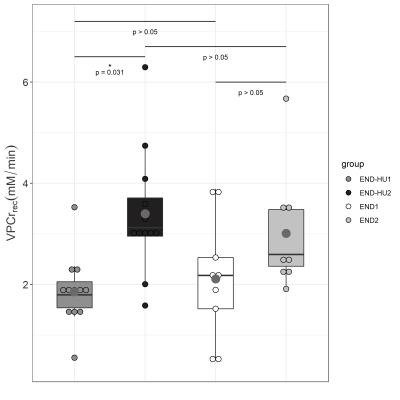
VPCrrec, the rate of PCr recovery, after the moderate exercise of each condition, before and after intervention. Results are presented in boxplot. Statistical results are displayed on the boxplot.
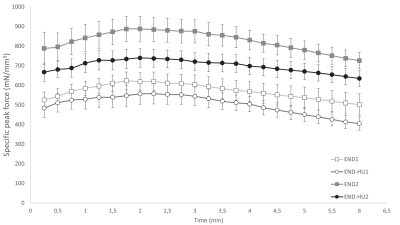
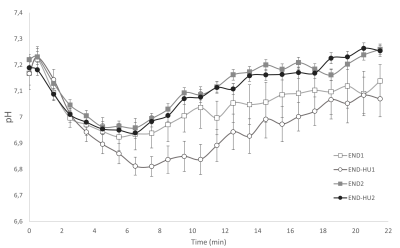
Time-dependant changes in specific peak force throughout the moderate exercise in each condition. Results are presented as mean ± SEM.