1137
Effect of Resistance Training on Lower-Extremity Gait Kinematics and Muscle Morphology
Michael Ko1, Andrew Schmidt1, Elka Rubin1, Lauren Watkins2, Marco Barbieri1, Laurel Hales3, Anthony Gatti1, Garry Gold1,2, Feliks Kogan1, Scott Delp2,4,5, Valentina Mazzoli1, and Akshay Chaudhari1,6
1Radiology, Stanford University, Stanford, CA, United States, 2Bioengineering, Stanford University, Stanford, CA, United States, 3Electrical Engineering, Stanford University, Stanford, CA, United States, 4Mechanical Engineering, Stanford University, Stanford, CA, United States, 5Orthopaedic Surgery, Stanford University, Stanford, CA, United States, 6Biomedical Data Science, Stanford University, Stanford, CA, United States
1Radiology, Stanford University, Stanford, CA, United States, 2Bioengineering, Stanford University, Stanford, CA, United States, 3Electrical Engineering, Stanford University, Stanford, CA, United States, 4Mechanical Engineering, Stanford University, Stanford, CA, United States, 5Orthopaedic Surgery, Stanford University, Stanford, CA, United States, 6Biomedical Data Science, Stanford University, Stanford, CA, United States
Synopsis
Gait retraining has been studied as an intervention to improve osteoarthritis symptoms but has a variety of limitations. An alternative approach may be muscle strengthening interventions that have known impacts on gait changes. However, limited studies have examined the quantitative relationships between muscle strengthening and gait. Here, we combine a rapid MRI protocol with wearable sensors to determine that a 12-week exercise intervention induced significant changes in both quadricep muscle morphology and gait kinematics. Morphological changes in different muscles were related to different kinematic changes, which may inform future strengthening interventions that aim to achieve specific changes in gait kinematics.
INTRODUCTION
Knee osteoarthritis (OA) is a degenerative joint disease frequently results in altered gait, reduced muscle strength, and lower quality of life1. Changing gait patterns has been studied as an intervention to improve OA symptoms2,3. However, assessing gait retraining accurately requires time-consuming biomechanical assessments in dedicated gait labs4. Gait retraining studies may also be limited due to a low compliance of new gait patterns when outside of a laboratory setting5. Muscle strengthening is another common method to reduce OA symptoms6,7, and an alternative approach to gait interventions may be prescribing specific muscle strengthening interventions that have known impacts on changes in gait. However, studies that examine quantitative relationships between muscle strengthening and gait changes are limited. In this study, to better evaluate structural-functional physiological changes, we examine the role of an exercise intervention on muscle morphology measured via a rapid MRI protocol and gait kinematics measured via portable wearable sensors.METHODS
Five healthy participants (3 female, age: 27±2 years, BMI: 23±1) participated in this study. All participants underwent an exercise intervention in which they performed three sets of 15 repetitions of unilateral squats with both legs on a 25º decline board (Fig.1). This exercise was performed twice daily, three-four times a week, for 12 consecutive weeks. The participants underwent an MRI scan the day before the start of the exercise protocol and at the conclusion of the 12-week exercise program.All MRI scans were acquired on a 3T MRI scanner (SIGNA 750w Premier, GE Healthcare). To assess muscle morphology, participants underwent a multi-stack upper leg bilateral scan with a flexible coil (Air coil, GE Healthcare) that included Dixon water/fat-separated images (3D GRE, matrix size=256x256x46, voxel size=1.56x1.56x5 mm3, TR=14 ms, TE=1.2 ms, 3min58s). The merged Dixon-Water stacks were used to manually segment the vastus medialis (VM), vastus lateralis (VL), and rectus femoris (RF) muscles from which muscle volume was calculated (Fig.1). All muscle volumes were normalized by participant weight to create weight-normalized muscle volumes (in cm3/kg).
Immediately following each MRI scan, all participants underwent a biomechanical assessment using portable wearable sensors (Cionic, San Francisco, CA). A total of five inertial measurement units (IMUs) were attached to each shank and thigh, and the central pelvis via adjustable straps (Fig.1). Participants walked overground for 20 seconds at a self-selected normal walking pace. The IMU data was filtered with a fourth-order low-pass Butterworth filter at 6Hz and used to calculate flexion-extension kinematics of the knee and the hip. Three kinematic features were extracted by calculating the 1) maximum flexion-extension angle, 2) area under the curve, and 3) absolute value of the curve of the kinematic data, according to prior studies8.
Paired t-tests were conducted to assess the change in muscle volume from pre-intervention (baseline) to post-intervention. Since each participant has multiple correlated measurements of kinematic data for both legs, linear mixed models were used to quantify change in kinematic features due to exercise intervention as well as relate muscle volumes to kinematic features.
RESULTS
The 12-week exercise intervention significantly increased right VL muscle volume (5.75 cm3/kg, p<.01) and marginally increased both right VM (2.42 cm3/kg, p=.07) and right RF muscle volume (2.56 cm3/kg, p=.06) (Table 1).Exercise intervention also significantly increased absolute area of hip flexion (2.59, p=.02) and knee flexion (1.79, p=.03) as well as area of knee flexion (1.65, p=.03). A marginal increase in peak hip flexion (5.34, p=.06) was also observed (Table 2).
Significant correlations using a linear mixed model were observed between muscle volume and kinematic features. VL volume was significantly positively associated with maximum angle (5.67, p=.02), area (4.81, p=.03), and absolute area (2.49, p=.02) of hip flexion and marginally with area (2.79, p=.09) and absolute area (2.89, p=.07) of knee flexion. VM volume was marginally negatively associated with maximum (-6.81, p<.01), area (-4.82, p=.02), and absolute area (-2.67, p=.01) of hip flexion. RF volume was negatively associated with the absolute area (-3.46, p=.07) of knee flexion (Table 3).
DISCUSSION
This study develops a proof-of-concept framework for assessing the relationship between multi-joint biomechanics and muscle morphology. With our multimodal assessment, we observed significant changes in both quadricep muscle morphology and hip and knee joint kinematics following a 12-week exercise intervention. In this pilot study, we found that 1) exercise-induced changes in the VM and VL muscles were related to different kinematic changes, 2) Increased VL volume was related to increased hip and knee flexion, and 3) Increased VM volume was related to decreased hip flexion. Future work with more participants will help establish improved knowledge regarding such structural-functional relationships. This preliminary study may inform future interventions that aim to achieve specific changes in gait kinematics.CONCLUSION
We demonstrated a proof-of-concept workflow through MRI and wearable sensors to characterize the relationship between exercise induced changes in quadriceps muscle morphology and hip and knee joint kinematics. Our preliminary results indicate changes in the morphology of different muscles result in changes to various kinematic outcomes, which may inform future work that defines causal relationships between muscle strengthening mechanisms to gait changes.Acknowledgements
Research support provided by NIH: R01 AR077604, R01 EB002524, and K24 AR062068, the Precision Health and Integrated Diagnostics at Stanford, Philips, GE Healthcare, Wu Tsai Human Performance Alliance, and Cionic.References
1. Vincent KR, Vincent HK. Resistance exercise for knee osteoarthritis. PM R. 2012;4(5 Suppl):S45-S52.2. Cheung, RTH, Ho KKW, Au IPH, et al. Immediate and short-term effects of gait retraining on the knee joint moments and symptoms in patients with early tibiofemoral joint osteoarthritis: a randomized controlled trial. Osteoarthritis and cartilage. 2018;(26)11:1479-1486.
3. Richards R, van den Noort JC, van der Esch M, et al. Gait retraining using real-time feedback in patients with medial knee osteoarthritis: Feasibility and effects of a six-week gait training program. The Knee. 2018; 25(5):814–824.
4. Toro B, Nester CJ, Farren PC. The status of gait assessment among physiotherapists in the United Kingdom. Arch Phys Med Rehabil. 2003;84(12):1878-1884.
5. Richards R, van den Noort JC, Dekker J, et al. Gait retraining with real-time biofeedback to reduce knee adduction moment: systematic review of effects and methods used, Arch Phys Med Rehabil. 2017;98(1):137-150.
6. Lange AK, Vanwanseele B, Fiatarone Singh MA. Strength training for treatment of osteoarthritis of the knee: a systematic review. Arthritis Rheumatol. 2008;59(10):1488–1494.
7. Kaufman KR, Hughes C, Morrey BF, et al. Gait characteristics of patients with knee osteoarthritis. J. Biomech. Journal of biomechanics. 2001;34(7):907–915.
8. Kwon SB, Ro DH, Song MK, et al. Identifying key gait features associated with the radiological grade of knee osteoarthritis. Osteoarthritis and cartilage. 2019;27(12):1755-1760.
Figures
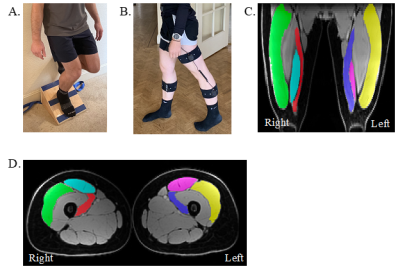
Figure 1: A. Example participant performing unilateral squat
exercise. B. Example participant wearing portable gait sensors used for gait
capture in this study. C. Segmented muscles (green/yellow = vastus lateralis
(VL), red/blue = vastus medialis (VM), teal/pink = rectus femoris (RF)) on an
example coronal Dixon-water upper leg bilateral image. B. Segmented muscles
(green/yellow = VL, red/blue = VM, teal/pink = RF) on an example axial
Dixon-water upper leg bilateral image.
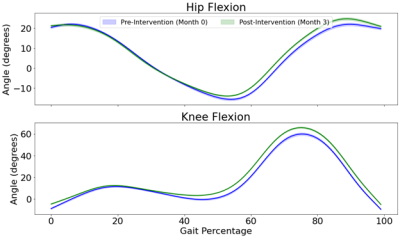
Figure 2: Example participant gait kinematics for hip and knee
flexion measured at baseline and month 3. A gait cycle begins at the heal
strike of one foot (0%) and continues until the heel strike of the same foot
for the next step (100%).
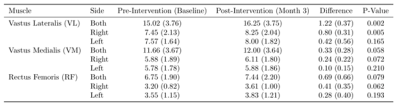
Table 1: Mean and standard deviation values of muscle volume. The
average post-intervention muscle volume was greater than baseline for all
muscle groups. Intervention induced greater muscle volume change for the right
leg compared to left.
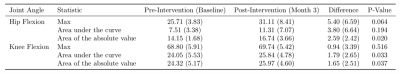
Table 2: Mean and standard deviation values of kinematic features.
Intervention induced a positive shift for all kinematic features of the hip and
knee flexion.

Table 3: Correlation coefficient extracted from linear mixed models
between kinematic features and muscle volume. † p < .1, * p < .05, ** p
< .01, *** p < .001
DOI: https://doi.org/10.58530/2022/1137