1127
Preservation of Brain Oxygen Metabolism in Emerging Young Adult Survivors of Fontan Palliation for Single Ventricle.1Bioengineering, University of Southern California, Los Angeles, CA, United States, 2Children's Hospital of Los Angeles-USC KSOM, Los Angeles, CA, United States, 3Neurosciences, University of Southern California, Los Angeles, CA, United States, 4Radiology, Children's Hospital Pittsburgh, Pittsburgh, PA, United States, 5Pediatrics, Children's Hospital of Los Angeles-USC KSOM, Los Angeles, CA, United States
Synopsis
Surgical palliation of single ventricular heart disease (Fontan procedure) leads to poor cardiac output, high central venous pressures, and long-term multiorgan morbidity. We report cerebral blood flow, brain oxygen delivery, and cerebral metabolic rate in 20 Fontan survivors and 9 control subjects. Fontan patients had lower cerebral blood flow and oxygen saturation but maintained cerebral oxygen delivery and cerebral metabolic rate of oxygen (CMRO2) because of polycythemia. Scattergram between CMRO2 and O2 delivery suggests similar oxygen extraction at the microvascular level in both groups. Taken together, these data suggest adequate cerebrovascular compensation to low cardiac output in Fontan patients.
Introduction
Single ventricular heart diseases are among the most complicated congenital heart defects to manage, typically requiring three surgeries to restore fully oxygenated blood flow to the body. The final stage, or Fontan palliation, requires the entire cardiac output to pass through the lungs without ventricular assist, leading to physiology similar to chronic right heart failure (low cardiac index and high central venous pressures). There is a paucity of prior work studying the impact of this physiology on brain health in young adults. We hypothesized that Fontan patients would have impaired oxygen delivery and increased brain oxygen extraction compared with age-matched control subjects.Methods
We prospectively recruited 20 Fontan patients and 9 control subjects from outpatient Fontan clinics. All Fontan patients were non-fenestrated and had no known source of right-to-left shunt. All patients underwent MRI on a 3T Philips Achieva D-Stream with a 32-element head coil. Complete blood count was obtained on the same day by phlebotomy. Arterial O2 saturation (SaO2) was measured using pulse oximetry. Cerebral blood flow (CBF) was measured by phase contrast measurements in the neck as previously described1,2 and normalized to age/sex corrected brain weights. T2 relaxation under spin tagging was measured in the sagittal sinus using standard techniques3 and converted to brain venous oxygen saturation (SvO2) using a calibration derived from human blood4. These metrics were used to calculated oxygen delivery, oxygen extraction fraction (OEF) and the cerebral metabolic rate of oxygen consumption (CMRO2) using the following, well-known, relationships:1) O2 delivery = 1.34 x Hemoglobin x SaO2 x CBF
2) OEF = (SaO2 - SvO2)/ SaO2
3) CMRO2 = O2 delivery x OEF
Results
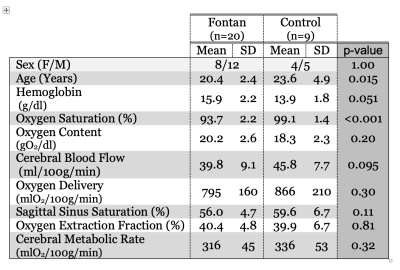
Patient demographics are summarized in Table 1. The control subjects averaged 3 years older than the Fontan population (p=0.015) but were balanced for sex (Table 1). Oxygen saturation was 5.4% (absolute saturation percent) lower in the Fontan population, and correlated with a compensatory increase in hemoglobin (2 g/dl). Cerebral blood flow (CBF) trended 15% lower in the Fontan population (p=0.095), however cerebral oxygen delivery was only 8% lower (p=0.3). Sagittal sinus oxygen saturation trended 3% lower in the Fontan population (3% lower, p=0.11), but OEF was nearly identical. As a result, there was no significant difference in CMRO2 between the two groups. Figure 1 demonstrates the relationship between CMRO2 and oxygen delivery in the two experimental groups. The solid line reflects the relationship derived in historical control subjects5. Both the current controls and the Fontan patients are symmetrically distributed about this relationship.Discussion
Even though the Fontan patients were unfenestrated, their oxygen saturations were more than 5% points lower, likely reflecting contributions of coronary sinus flow as well as poor ventilation perfusion matching in the lung. The resulting cyanosis was likely the stimulus for the polycythemia observed in the Fontan population. Interestingly, the oxygen content was higher in the Fontan population, suggesting either overcompensation of the polycythemia or that the saturations recorded at the time of the MRI underestimated the time-averaged hypoxic burden during an entire day.The decreased CBF observed in Fontan patients is almost certainly secondary to their polycythemia, through one of two mechanisms. Oxygen content is the strongest single predictor of CBF, creating a strong inverse relationship between CBF and oxygen content1,2 that preserves cerebral oxygen delivery over a broad range of hemoglobin levels. Alternatively, blood viscosity is directly proportional to hemoglobin and hematocrit levels. As a result of their polycythemia, Fontan patients could be operating in an unfavorable balance of oxygen content and viscosity6.The lower oxygen saturation in the sagittal sinus for the Fontan patients was not surprising given that that systemic oxygen saturation was decreased as well. In fact, the oxygen extraction fraction and the overall relationship of CMRO2 to oxygen delivery were quite similar to control subjects, suggesting intact brain microvascular oxygen extraction mechanisms.Conclusion
Taken together, these data suggest adequate cerebrovascular compensation, under resting conditions, to the low cardiac output and high central venous pressures observed in Fontan patients. Further work is ongoing to characterize vascular reactivity and blood-brain barrier permeability as well as acquired brain injury and cognitive outcomes in this population. Larger sample sizes refine these initial observations and account for inter-subject differences in disease severity.Acknowledgements
This work was supported by the Additional Ventures Single Ventricle Fund, National Heart, Lung, and Blood Institute (grant 1U01-HL-117718-01, 1R01-HL136484-01A1), and the National Center for Research (5UL1-TR000130-05) through the Clinical Translational Science Institute at Children’s Hospital Los Angeles. Philips Healthcare provided support for protocol development and applications engineering on a support-in-kind basis.References
1. Borzage MT, Bush AM, Choi S, et al. Predictors of cerebral blood flow in patients with and without anemia. J Appl Physiol (1985) 2016;120:976-81.
2. Bush AM, Borzage MT, Choi S, et al. Determinants of resting cerebral blood flow in sickle cell disease. Am J Hematol 2016;91:912-7.
3. Bush AM, Coates TD, Wood JC. Diminished cerebral oxygen extraction and metabolic rate in sickle cell disease using T2 relaxation under spin tagging MRI. Magn Reson Med 2018;80:294-303.
4. Bush A, Borzage M, Detterich J, et al. Empirical model of human blood transverse relaxation at 3 T improves MRI T2 oximetry. Magn Reson Med 2017;77:2364-71.
5. Vu C, Bush A, Choi S, et al. Reduced global cerebral oxygen metabolic rate in sickle cell disease and chronic anemias. Am J Hematol 2021;96:901-13.
6. Detterich J, Alexy T, Rabai M, et al. Low-shear red blood cell oxygen transport effectiveness is adversely affected by transfusion and further worsened by deoxygenation in sickle cell disease patients on chronic transfusion therapy. Transfusion 2013;53:297-305.