1120
Static and dynamic parallel transmission (pTx) for human cardiac MRI at 14.0 T1Berlin Ultrahigh Field Facility (B.U.F.F.), Max-Delbrück Center for Molecular Medicine in the Helmholtz Association, Berlin, Germany, 2Technische Universität Berlin, Chair of Medical Engineering, Berlin, Germany, 3Physikalisch-Technische Bundesanstalt (PTB), Braunschweig and Berlin, Germany, 4MRI.TOOLS GmbH, Berlin, Germany, 5Experimental and Clinical Research Center (ECRC), a joint cooperation between the Charité Medical Faculty and the Max-Delbrück Center for Molecular Medicine, Berlin, Germany
Synopsis
Transmission field inhomogeneities at ultrahigh and extreme field MRI can be offset by using static or dynamic pTx. Responding to the challenges and recognizing the opportunities of cardiac MRI, this abstract examines the feasibility of parallel transmission (pTx) using fractionated dipole (FRD) RF array configurations for static and dynamic B1+ homogenization of the heart at 7.0T and 14.0T. Our results reveal that static pTx provides limited performance at 14.0 T but dynamic pTx enables uniform excitation of the heart at 14.0T. This finding is heartening and provides the technical foundation for explorations into cardiac MRI at 14.0T.
Introduction
MRI of the human torso and heart at ultrahigh magnetic fields (UHF, B0 ≥ 7.0T) benefits from the sensitivity gain and spatial resolution.1,2 This benefit is challenged by transmission field (B1+) inhomogeneities due to RF wavelength shortening and destructive/constructive electromagnetic field (EMF) interferences. For cardiac UHF-MRI (CMRI) the highly heterogeneous tissue environment within the thorax constitutes an extra challenge. To mitigate spatial B1+ variation local RF transceiver arrays2,3 were established to support B1+ shimming, through an adaptation of multichannel transmission.4 Pushing the boundaries and unlocking the potential of extreme field MR (EF, B0 ≥ 14.0T) requires unraveling and leveraging the electrodynamics of the short wavelength regime. To respond to the challenges and in recognition of the opportunities of CMRI at 14.0T, this abstract elucidates the electrodynamic constraints at high spin excitation frequencies, explores the benefits of multi-transmission using a fractionated dipole (FRD) RF array, and demonstrates the feasibility of static and dynamic parallel transmission (pTx) using optimized kT points.4–6Methods
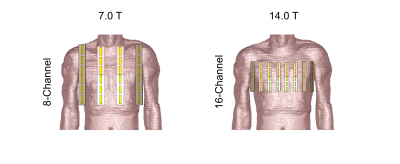
The 7.0 T FRD cardiac array was reproduced according to previous reports of this antenna type for CMRI.7,8 The meander elements were modeled by lumped elements and designed to enhance the antenna’s performance according to the design recommendations.7 With increasing static magnetic field strength the antenna dimensions were scaled respectively to the wavelength and electrically optimized. The inductivity was set to minimize the imaginary part of the antenna’s impedance and additionally represent a trade-off between superficial SAR and B1+ efficiency (B1+ scaled to √1 kW input power). At 7.0 T an 8-channel and at 14.0 T a 16-channel configuration were investigated (Figure 1). EMF simulations were performed in CST Microwave Studio (CST Studio Suite 2020, Dassault Systèmes, Vélizy-Villacoublay Cedex, France) using the human voxel model Duke9 at a resolution of 1.0x1.0x1.0 mm³. The mesh size of the voxel model was kept at 4.0x4.0x4.0 mm³. Post-processing was performed in Matlab 2019b (MathWorks, Natick, MA) including tuning and matching and channel-wise B1+ calculation. For the static pTx approach (i.e. B1+ shimming) a genetic algorithm (GA) of the Matlab global optimization toolbox was used. The static pTx approach consists of one rectangular RF pulse with 1ms pulse duration. The RF pulse was optimized to (i) minimize the spatial B1+ variation (Coefficient of Variation, CoV(B1+)=SD/mean) and (ii) to maximize the minimum B1+ efficiency in the 3D volume of the heart (Region Of Interest, ROI). For dynamic pTx several RF sub-pulses were used for parallel transmission - separated by gradient blips – with the goal of 3D flip angle (FA) homogenization (CoV(FA)) targeting the whole heart. The pulse design6,10,11 was performed in Matlab using the small-tip-angle approximation (STA)12 for a nominal FA distribution of 10° in the heart. At 14.0 T 4, 8, and 12 kT points were optimized with a total pulse duration of 0.96 ms, 1.92 ms, and 2.88 ms respectively.Results
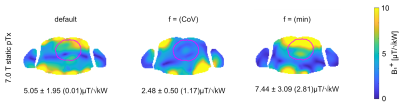
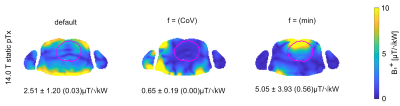
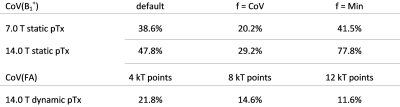
B1+ distributions obtained for static pTx using the FRD RF array configurations at 7.0 T and 14.0 T are shown in Figure 2 and 3. For the default setting (equal amplitude and phase for each channel) the mean B1+ efficiency obtained for the cardiac ROI at 14.0 T is reduced by 50% and the CoV(B1+) is increased by 9% versus the 7.0 T reference. GA optimization based static pTx using the cost function CoV(B1+) revealed for the cardiac ROI: CoV of 20.2% (7.0 T) and 29.2% (14.0 T). Min(B1+) was 1.17 µT/√kW at 7.0 T and 0 µT/√kW at 14.0 T. For GA optimization using the cost function min(B1+) CoV was 41.5% (7.0 T) and 77.8% (14.0 T). Min(B1+) was 2.81 µT/√kW at 7.0 T and 0.56µT/√kW at 14.0 T. Figure 4 highlights the results obtained from dynamic pTx with 4, 8, and 12kT points. Increasing the kT point count resulted in an improved FA homogeneity which is expressed by a CoV(FA) of 21.8%, 14.6%, and 11.6%. The CoV deduced for the cardiac ROI is summarized in Table 1 for static and dynamic pTx.Discussion and Conclusion
Our EMF simulations demonstrate the B1+-uniformity and efficiency challenges of CMRI at 14.0 T. At 7.0 T these challenges can be addressed with the static pTx. When moving to B0=14.0 T static pTx is no longer sufficient to overcome B1+ inhomogeneities. Our findings demonstrate that kT-point pTx pulses are highly suitable for mitigating FA inhomogeneities for a 3D cardiac ROI at 14.0 T. For STA the FA scales linearly with B1+ and therefore the CoV(FA) of the dynamic pTx can be benchmarked against the CoV(B1+) of the static pTx. Dynamic pTx showed improved CoV compared to the static pTx at 14.0 T. The improved CoV with increasing kT points results in a more homogeneous FA distribution but will come at the cost of reduced B1+ efficiency due to the longer pulse durations.To conclude, static pTx provides limited performance at 14.0T. Dynamic pTx enables uniform excitation of the heart at 14.0T. This finding is heartening and provides the technical foundation for explorations into cardiac MRI at extreme field strength.
Acknowledgements
This project has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program under grant agreement No 743077 (ThermalMR).References
1. Vaughan JT, Garwood M, Collins CM, et al. 7T vs. 4T: RF power, homogeneity, and signal-to-noise comparison in head images. Magn Reson Med. 2001;46(1):24-30. doi:10.1002/mrm.1156
2. Niendorf T, Graessl A, Thalhammer C, et al. Progress and promises of human cardiac magnetic resonance at ultrahigh fields: A physics perspective. J Magn Reson. 2013;229:208-222. doi:10.1016/j.jmr.2012.11.015
3. Zhu Y. Parallel Excitation with an Array of Transmit Coils. Magn Reson Med. 2004;51(4):775-784. doi:10.1002/mrm.20011
4. Padormo F, Beqiri A, Hajnal J V., Malik SJ. Parallel transmission for ultrahigh-field imaging. NMR Biomed. 2016;29(9):1145-1161. doi:10.1002/nbm.3313
5. Aigner CS, Dietrich S, Schaeffter T, Schmitter S. Calibration-free pTx of the human heart at 7T via 3D universal pulses. Magn Reson Med. 2021;(July):1-15. doi:10.1002/mrm.28952
6. Aigner CS, Dietrich S, Schmitter S. Three-dimensional static and dynamic parallel transmission of the human heart at 7 T. NMR Biomed. 2021;34(3):1-15. doi:10.1002/nbm.4450
7. Raaijmakers AJE, Italiaander M, Voogt IJ, et al. The fractionated dipole antenna: A new antenna for body imaging at 7 Tesla. Magn Reson Med. 2016;75(3):1366-1374. doi:10.1002/mrm.25596
8. Steensma BR, Voogt IJ, Leiner T, et al. An 8-channel Tx/Rx dipole array combined with 16 Rx loops for high-resolution functional cardiac imaging at 7 T. Magn Reson Mater Physics, Biol Med. 2018;31(1):7-18. doi:10.1007/s10334-017-0665-5
9. Christ A, Kainz W, Hahn EG, et al. The Virtual Family - Development of surface-based anatomical models of two adults and two children for dosimetric simulations. Phys Med Biol. 2010;55(2). doi:10.1088/0031-9155/55/2/N01
10. Grissom WA, Khalighi M-M, Sacolick LI, Rutt BK, Vogel MW. Small-tip-angle spokes pulse design using interleaved greedy and local optimization methods. Magn Reson Med. 2012;68(5):1553-1562. doi:10.1002/MRM.24165
11. Cao Z, Yan X, Grissom WA. Array-compressed parallel transmit pulse design. Magn Reson Med. 2016;76(4):1158-1169. doi:10.1002/MRM.26020
12. Pauly JM, Nishimura DG, MacOvski A. Introduction to: A k-space analysis of small-tip-angle excitation. J Magn Reson. 2011;213(2):558-559. doi:10.1016/j.jmr.2011.08.008
Figures