1118
Translating 3D Whole Heart LGE to Clinical Practice; Early Feasibility Results in a Tertiary UK Centre1Cardiology and Cardiac Surgery, Nottingham University Hospitals NHS Trust, Nottingham, United Kingdom
Synopsis
Accurately identifying left ventricular fibrosis is paramount for diagnostic, prognostic and procedural reasons. Increasing evidence for the benefit of 3D whole heart imaging in fibrosis identification is emerging. Limitations to integrating 3D whole heart imaging into routine practice include risking suboptimal imaging incumbering diagnostic value. We undertook retrospective quantitative and qualitative analysis of clinically indicated paired 2D and 3D imaging. Quantitative SNRscar and CNRscar-myo analysis was not significantly different and qualitative diagnostic utility was comparable both in patients with ischaemic and non-ischaemic disease. This small study provides evidence for routine usage of 3D whole heart imaging for scar identification.
Introduction
Accurate scar delineation and measurement carries importance for cardiac procedures, guiding decisions and judging prognosis1,2. Free-breathing 3D Whole-heart-imaging (3D-WHI) provides greater spatial resolution of scar with greater ventricular coverage and comparable image quality in research settings while aiming to reduce patient discomfort3,4. Specifically for electrophysiological procedures, 3D-WHI allows for the assessment of complex scar architecture to be displayed intra-procedurally and have correlated scar location with electrically important channels for targeting.However, “real-world” clinical data regarding 3D-WHI is sparse and scans in comorbid patients may demonstrate lower signal-noise-ratio (SNR) and contrast-noise-ratio (CNR) that could impede its diagnostic utility5.
We present early feasibility data of 3D-WHI late-gadolinium (LGE) sequence integration into clinical NHS practice for identification of potential arrhythmic substrate.
Methods
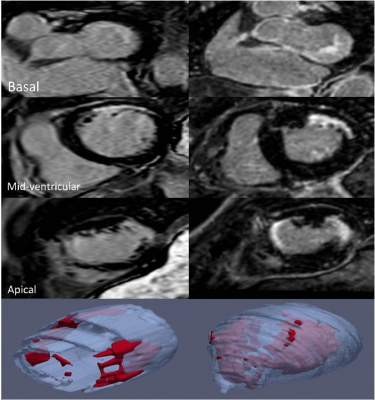
A retrospective analysis of routine clinical scans with paired 2D LGE (voxel size 0.89 × 0.89 × 10mm) and 3D-WHI (voxel size 0.94 × 0.94 × 1mm) sequences in those with confirmed/suspected ischaemic heart disease since sequence inclusion in late 2019 was undertaken. Paired images were acquired on a 1.5T Philips Ingenia® during the same-sitting starting with 2D LGE. 3D images were reformatted as a short axis image using original dimensions of 1mm slice thickness and no slice gaps for comparison with 2D LGE. Quantitative assessment was undertaken with SNR and CNR calculation of blood, scar and myocardium and compared using Wilcoxon signed rank test.Diagnostic utility was assessed qualitatively. Based upon clinical reports with additional image review (if not described), images were scored taking into account scar location and transmurality (0=non diagnostic, 1=limited value, 2=good value).
Results
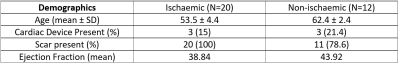
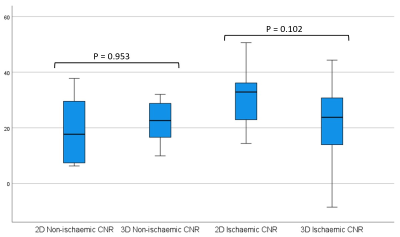
32 paired images were assessed: 20 ischaemic, 12 non-ischaemic (3 with no LGE). Cohort demographics can be seen in figure 1. 6 had implantable cardiac devices divided equally between the ischaemic and non-ischaemic cohorts, all implantable cardioverter defibrillators (ICD). Of these 6, there was only 1 uninterpretable study due to widespread artefact present in both 2D LGE and 3D-WHI sequences. No non-device images were uninterpretable. 4 had previous cardiac surgery – 2 coronary artery bypass graft, 1 aortic valve replacement and 1 ventricular septal defect repair.There was no significant difference between 2D and 3D-WHI cohorts for CNRscar-myo (28 vs. 21.85) or SNRscar (30.46 vs. 26.09) but there was a significant difference in SNRmyo (3.53 vs. 4.81, p<0.01) and SNRblood (31.82 vs. 20.11, p<0.01). When subdivided by overarching diagnosis, 2D LGE had a significantly higher SNRblood in the ischaemic cohort and lower SNRmyo in the non-ischaemic cohorts. Similar trends were seen in the opposing diagnoses, though not statistically significant (figure 2).
The cardiac devices cohort displayed no significant difference between 2D LGE and 3D-WHI for CNRscar-myo (16.88 vs. 22.57) or SNRscar (18.59 vs. 25.19).
Qualitatively, imaging was described as “good” for scar location and transmurality for 22/ 32 (68.75%) and 27/32 (84.38%) of the 2D and 3D WHI cohorts respectively.
Discussion
Our local data demonstrates that integration of diagnostic free-breathing 3D-WHI into standard clinical care is feasible for those undergoing routinely indicated scans with comparable CNRscar-myo and SNRscar to standard 2D imaging. This applies to both ischaemic and non-ischaemic aetiologies.3D-WHI SNRmyo was higher comparatively to 2D LGE likely due to longer acquisition times and, subsequently, varying TI. This could result in decreased appropriate identification of fibrotic tissue and this should theorectically result in a similar ability for clinicians to identify scar. However, our data did not show a significant difference in the CNRscar-myo suggesting minimal impact on scar recognition. This quantitative evaluation of scar was borne out in the qualitative scar assessment with distribution and diagnostic quality similar between cohorts. Furthermore, the decreased SNRblood seen may be mitigated with the 3D-WHI undertaken prior to 2D LGE though previous data demonstrated similar results despite sequence order randomisation5. Other advanced techniques to minimise acquisition time such as compressed sensing or progressive TI altering may result in a more nulled myocardium6.
Conclusion
Whilst a small cohort, this data provides supporting evidence that the routine use of 3D-WHI for fibrosis identification may not necessarily be hindered clinically by image quality whilst providing greater spatial resolution important in procedural planning and navigation. Further studies assessing the impact on workflow are required in particular relating to acquisition time with recent advances in compressed sensing.Acknowledgements
No acknowledgement found.References
1. Andreu D, Penela D, Acosta J, et al.: Cardiac magnetic resonance-aided scar dechanneling: Influence on acute and long-term outcomes. Heart Rhythm 2017; 14:1121–1128. Available from: http://dx.doi.org/10.1016/j.hrthm.2017.05.018
2. Wong TC, Piehler KM, Zareba KM, et al.: Myocardial damage detected by late gadolinium enhancement cardiovascular magnetic resonance is associated with subsequent hospitalization for heart failure. J Am Heart Assoc. Wiley-Blackwell, 2013; 2. Available from: /pmc/articles/PMC3886781/?report=abstract
3. Bizino MB, Tao Q, Amersfoort J, et al.: High spatial resolution free-breathing 3D late gadolinium enhancement cardiac magnetic resonance imaging in ischaemic and non-ischaemic cardiomyopathy: quantitative assessment of scar mass and image quality. Eur Radiol Springer, 2018; 28:4027. Available from: /pmc/articles/PMC6096581/
4. Jablonowski R, Nordlund D, Kanski M, et al.: Infarct quantification using 3D inversion recovery and 2D phase sensitive inversion recovery; validation in patients and ex vivo. BMC Cardiovasc Disord. BioMed Central, 2013; 13:110. Available from: https://bmccardiovascdisord.biomedcentral.com/articles/10.1186/1471-2261-13-110
5. Bratis K, Henningsson M, Grigoratos C, et al.: Image-navigated 3-dimensional late gadolinium enhancement cardiovascular magnetic resonance imaging: Feasibility and initial clinical results. J Cardiovasc Magn Reson BioMed Central Ltd., 2017; 19:97. Available from: https://jcmr-online.biomedcentral.com/articles/10.1186/s12968-017-0418-7
6. Holtackers RJ, Gommers S, Van De Heyning CM, et al.: Steadily Increasing Inversion Time Improves Blood Suppression for Free-Breathing 3D Late Gadolinium Enhancement MRI With Optimized Dark-Blood Contrast. Invest Radiol 2020; Publish Ah:1–6
Figures