1117
Investigation of Spatial-Spectral Selective Pulses for B1 Compensation and Improved Saturation Homogeneity in Cardiac CEST MRI1Department of Bioengineering, University of California, Berkeley, Berkeley, CA, United States, 2Biomedical Engineering, Vanderbilt University, Nashville, TN, United States
Synopsis
Chemical Exchange Saturation Transfer (CEST) MRI is highly susceptible to B1 inhomogeneities, resulting in inconsistent contrast for tissues with equal metabolite concentrations. To enhance B1-uniformity of saturation across the myocardium, a spectral-spatially selective pulse for CEST preparation was tailored for each subject. Simulations showed the tailored pulse reduced B1 variation from 6.85⁰ to 2.9⁰. These results were investigated on a Siemens 3T Trio scanner by performing full cardiac CEST exams using a conventional Gaussian or a tailored pulse for saturation. The tailored pulse resulted in reduced water and MT contrast variation across the myocardium when compared to the conventional pulse.
Introduction
Chemical exchange saturation transfer (CEST) enables the detection of certain low-concentration metabolites1 but is highly sensitive to B1-field inhomogeneities. Current efforts to improve the accuracy of CEST include mitigating these B1 inhomogeneities2. However, methods to reduce B1 inhomogeneity in stationary organs using parallel transmit head coils3 or specific post-processing correction that make assumptions upon anatomical scanning location4 are not applicable for cardiac imaging. Here, we evaluate the efficacy of tailored spectral-spatial pulses for CEST contrast generation to enhance spatial B1 homogeneity across the heart and improve consistency of CEST contrast generation.Methods
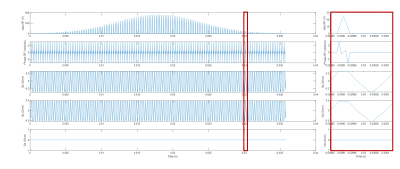
All in vivo studies were performed on a Siemens 3T Tim Trio scanner using a body matrix coil and a spine matrix coil. Two healthy subjects have been recruited to date for this study. A B1 map was acquired in one midventricular short axis slice using a Turbo FLASH sequence with a prescribed saturation flip angle of 50⁰. Additional parameters included a field of view (FOV) of 24.3 cm × 30 cm, slice thickness of 8mm, TE of 2.12ms, and TR of 2.73s.Using the measured B1 map for each subject, a subpulse was created with a maximum duration of 250 µs and design parameters consistent with scanner limitations. The spatial-spectral pulse was designed following previously described methods 5 using MATLAB version 2021b and then uploaded onto the scanner. A train of optimized sub-pulses was then created to span 36 ms and create the full RF pulse, illustrated in Figure 1. Finally, the pulse was fit to a Gaussian envelope and scaled to a peak B1 power of 1.2 µT. A simulated B1 map using the tailored RF pulse was also generated using the methods described previously5.
The cardiac CEST imaging sequence utilized 23 saturation pulses, each with a duration of 36 ms,1.2 µT peak B1 power, saturation offset ranging from -10ppm to +10ppm, and were followed by a 25⁰ flip angle GRE readout. Other scanning parameters included a FOV of 24.3 cm × 30 cm, TR of 1.45 s, and TE of 2.59 ms. Both saturation and readout utilized ECG triggering to time readout during diastole. Every scanning subject required two sets of frequency offset acquisitions: each set utilizing either a conventional Gaussian or tailored pulse. A reference scan was taken at 10000 Hz. Saturation offsets were selected for denser sampling around the chemical shifts of amide and creatine at 3.2 and 1.9 ppm, respectively.
For each image, the myocardium was segmented into 6 regions consistent with current standards6. The mean signal intensity within each segment was normalized and the resulting z-spectrum was corrected for B0 inhomogeneities using a water saturation shift referencing (WASSR) correction7. CEST contrast was quantified by performing a 4-pool Lorentzian fitting of creatine, amide, water, and MT.
Results
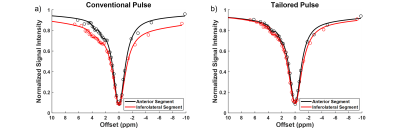
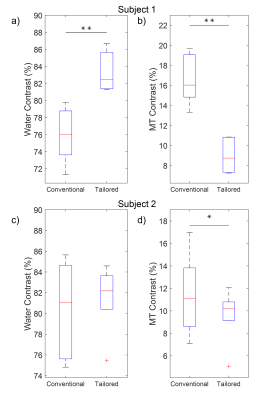
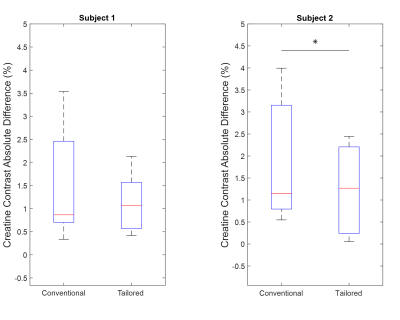
The use of spectral spatial sub-pulses reduced B1 variation across the myocardium from an average of 17.5% with a Gaussian pulse to 5.1% as shown in Figure 2. The subsequent impact upon Z-spectra derived from different myocardial regions is shown in Figure 3. Whereas the use of Gaussian pulses for CEST preparation resulted in significant regional variation in derived Z-spectra, spectral spatial saturation pulse design generated consistent Z-spectra across the heart. The subsequent impact on highly B1-sensitive parameters of direct saturation and MT is shown in Figure 4, with spectral spatial saturation resulting in significantly lower spatial variation of both parameters for subject 2. To determine the coherence of the creatine contrast, each segment was compared to a reference segment, the anteroseptal segment, shown in Figure 5. For both subjects, the tailored pulse saturation substantially reduced the spatial variation of creatine contrast.Discussion
The average percent error of 17.5% in the experimental B1 maps demonstrate the effects of B1 inhomogeneity in cardiac scans. The B1 map illustrates the flip angle’s spatial dependence which would result in inconsistent generation of CEST contrasts across the myocardium. The results of the simulated B1 map demonstrates an improved B1 homogeneity of 12.4% with the tailored RF pulse. Importantly, the use of a tailored RF pulse for generation of CEST contrast manifests in improved spatial homogeneity of multiple confounding contrast mechanisms. First, the span of water and MT contrast across all segments in Figure 4c and 4d represents a more consistent impact upon direct saturation and MT across the heart, reducing the potential for inaccurate quantification of subsequent creatine contrast. Figure 5 highlights this reduced creatine contrast spatial variation for both subjects with the tailored RF pulse. These preliminary findings suggest an improved saturation profile by using a tailored pulse, which will be further investigated with a larger sample size.Conclusion
This work explored the use of tailored spectral-spatial pulses for in vivo application and showed that, when compared to conventional Gaussian saturation pulse, the tailored pulse reduced the flip angle error by 12.4% on average. Preliminary results verify that the tailored pulse reduced creatine CEST contrast variation across the myocardium for both subjects, but only lowered the spatial variation of the direct saturation and MT signals in subject 2. Future work requires an increased sample size of cardiac CEST scans to explore the validity of the tailored spectral-spatial pulse.Acknowledgements
This study was supported by NIH 1R01HL28592-01, Silvian Foundation Award, AHA 19TPA34850040, NIH UH2EB028908-02, DGE 1752814
References
1. Wu B, Warnock G, Zaiss M, et al. An overview of CEST MRI for non-MR physicists. EJNMMI Phys. 2016;3(1):19. doi:10.1186/s40658-016-0155-2Figures