1108
Endogenous assessment of myocardial injuries using T1-rho mapping: comparison to T1 mapping, T2 mapping and late gadolinium enhancement imaging1IHU LIRYC, Electrophysiology and Heart Modeling Institute, Université de Bordeaux – INSERM U1045, Bordeaux, France, 2Department of Cardiovascular Imaging, Hôpital Cardiologique du Haut-Lévêque, CHU de Bordeaux, Bordeaux, France, 3Department of Diagnostic and Interventional Radiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 4Department of Cardiac Electrophysiology, Hôpital Cardiologique du Haut-Lévêque, CHU de Bordeaux, Bordeaux, France, 5CIBM Center for Biomedical Imaging, Lausanne, Switzerland
Synopsis
Myocardial T1-rho (T1ρ) mapping is a promising technique which reveals new insights about the macromolecular content of biological tissues. The idea that T1ρ mapping can be used to quantify myocardial fibrosis without contrast agent opens the door to new clinical capabilities. Yet, the tissue determinants driving T1ρ changes are still unclear, and the applicability of the technique to the broad spectrum of myocardial injuries remains uncharted territory. In the present study, we aim to identify clinical correlates of myocardial T1ρ and to examine how myocardial T1ρ values change under various clinical scenarios.
INTRODUCTION
Magnetic resonance myocardial T1-rho (T1ρ) mapping has emerged as a promising tool for detecting myocardial injuries without exogenous contrast agents.1,2,3 Yet, the tissue determinants driving T1ρ changes remain unclear, and the applicability of the technique to the broad spectrum of acute and chronic myocardial injuries uncharted territory. The aims of this study were to identify clinical correlates of myocardial T1ρ, and to examine how myocardial T1ρ values change under various clinical scenarios.METHODS
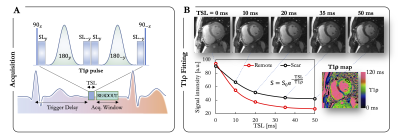
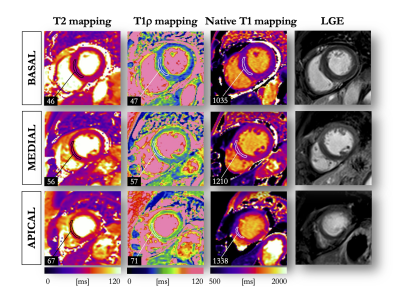
Image Acquisition: A total of 113 subjects were enrolled between October 2020 and December 2020. The population comprised 69 patients (51 males, age 54±16yo) with known ischemic (N=18) and non-ischemic (N=51) heart disease and 44 healthy controls (22 males, age 44±16yo). CMR was performed using a 1.5T Siemens MAGNETOM Aera scanner. Breath-held T1ρ mapping (pre-contrast, Fig.1), conventional T2 mapping (T2-prepared bSSFP, pre-contrast), T1 mapping (MOLLI, pre- and post-contrast), extracellular volume (ECV) quantification, and late gadolinium enhancement (LGE, 15min after injection of 0.2mmol/kg gadoteric acid) were performed in short-axis.2Image Analysis: Injured areas were defined by an expert as regions with LGE, while remote areas were defined as regions with no LGE. The agreement between T1ρ map, T2 map, ECV and LGE was assessed at a segmental level on the left ventricle (LV). In healthy subjects, relations of T1ρ with age, native T1 and T2 were compared using Pearson’s r correlation coefficient. In patients, quantitative analysis was achieved by tracing regions of interest on T1ρ, T2, and ECV maps within remote and injured areas. Paired t-tests were used to compare T1ρ measurements. All statistical tests were 2-tailed, with p values <0.05 considered to indicate statistical significance.
RESULTS
In healthy controls, mean myocardial T1ρ was 47±2ms. Compared to males, females had higher T1ρ (48±2ms vs. 46±2ms, P<0.01), T2 (47±2ms vs. 45±3ms, P=0.03) and ECV (26±2% vs. 24±2%, P<0.01). T1ρ correlated positively with age (R=0.4, P=0.003) and T2 (R=0.6, P<0.001), and significantly increased from basal to apical LV levels (P<0.01). In patients with acute focal myocardial injuries (N=9 with focal T2 elevation), T1ρ significantly increased (69±7ms vs. 48±2ms in remote, P<0.01). In patients with focal fibrosis (N=27 with positive LGE and negative T2), T1ρ significant increased (64±5ms vs. 47±2ms in remote, P<0.01). In patients with diffuse myocardial involvement (N=8 with ECV>30%), T1ρ significantly increased (52±6ms vs. 47±2 in controls, P<0.01) with a moderate positive correlation with ECV (R=0.3, P<0.01). Examples of patients with ischemic and non-ischemic cardiomyopathies are shown in Figures 2 and 3.CONCLUSION
Myocardial T1ρ values are gender and age dependent and are slightly higher in the apical segments. The technique appears to be equally sensitive to acute, chronic, focal, and diffuse myocardial injuries, and may thus be a contrast-free adjunct to LGE for gaining new and quantitative insight into acute and chronic myocardial structural disorders.Acknowledgements
This research was supported by funding from the French National Research Agency under grant agreements ANR-21-CE17-0034-01, Equipex MUSIC ANR-11-EQPX-0030 and Programme d’Investissements d’Avenir ANR-10-IAHU04-LIRYC, and from the European Council under grant agreement ERC n715093. AB acknowledges a Lefoulon-Delalande Foundation fellowship administered by the Institute of France.References
1. Witschey WE, et al. In vivo chronic myocardial infarction characterization by spin locked cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 14, 37 (2012). https://doi.org/10.1186/1532-429X-14-37.2.
2. Bustin A, et al. Endogenous assessment of myocardial injury with single-shot model-based non-rigid motion-corrected T1 rho mapping, J. Cardiovasc. Magn. Reson. 23, 119 (2021). https://doi.org/10.1186/s12968-021-00781-w.
3. Van Oorschot JWM, et al. Single breath-hold T1r -mapping of the heart for endogenous assessment of myocardial fibrosis. Invest. Radiol. 51(8):505-12 (2016). https//doi.org/10.1097/RLI.0000000000000261.
Figures