1106
Improved high-resolution fMRI image quality with simultaneous multislice VFA-FLEET using a novel multi-kernel slice-GRAPPA algorithm1Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 2Department of Radiology, Harvard Medical School, Boston, MA, United States, 3Department of Radiology, Stanford University, Palo Alto, CA, United States, 4Department of Electrical Engineering, Stanford University, Palo Alto, CA, United States, 5Q Bio, Inc., San Carlos, CA, United States, 6Vanderbilt University Institute of Imaging Science, Vanderbilt University, Nashville, TN, United States, 7Department of Biomedical Engineering, Vanderbilt University, Nashville, TN, United States, 8Harvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA, United States
Synopsis
We propose a novel slice-GRAPPA reconstruction algorithm, termed multi-kernel slice-GRAPPA (mks-GRAPPA), to tackle the challenge of reconstructing high spatial resolution segmented multi-shot EPI data for fMRI. This is particularly relevant for the recently proposed Variable-Flip-Angle “FLEET” pulse sequence. For a segmentation factor, S, by training 2×S slice-GRAPPA kernels, rather than one, we demonstrate significant improvements in image quality metrics under a wide range of protocols. The multitude of kernels account for static signal discontinuities within and across segments in multi-shot EPI. In the SNR starved regime of high-res fMRI, mks-GRAPPA allows us to recover a significant portion of lost tSNR.
Introduction
Performing fMRI at submillimeter spatial resolution requires increased encoding in k-space. For echo-planar imaging (EPI), this encoding prolongs the echo-train duration and minimum echo-time, resulting in spatial blur, geometric distortion, and reduced functional sensitivity. Due to gradient hardware performance limitations, peripheral nerve stimulation constraints1, and limits on acceptable undersampling factors for parallel imaging, we are currently encoding limited, demanding alternative encoding strategies2,3.Segmented EPI readout can help overcome the image-encoding burden4. Conventional segmentation has prolonged delays between acquiring the segments comprising a slice, making it vulnerable to intermittent ghosting artifacts. The Variable-Flip-Angle Fast Low-angle Excitation Echo planar Technique (VFA-FLEET) pulse sequence5,6, where segments of a given slice are acquired sequentially7, can be used to perform high-resolution fMRI with minimal intermittent ghosting. However, for high-resolution imaging, more slices are required to achieve acceptable slice coverage, resulting in reduced temporal efficiency, which can be compounded in multi-shot EPI.
Simultaneous multislice (SMS) imaging using slice-GRAPPA8 can boost the temporal efficiency of fMRI9–12. Due to the misalignment of alternating k-space lines in single-shot EPI, using distinct slice-GRAPPA kernels for readout positive and negative lines has been shown to reduce artifact levels compared to using a single-kernel reconstruction13. In multi-shot interleaved EPI, however, the pattern of misaligned k-space lines no longer simply alternates across consecutive lines, thus preventing the straightforward application of the two-kernel method.
We propose a generalization of the two-kernel slice-GRAPPA algorithm for multi-shot EPI data, referred to here as multi-kernel slice-GRAPPA (mks-GRAPPA), to account for static phase and magnitude differences across readout polarities and shots. mks-GRAPPA is compared against the single-kernel slice-GRAPPA algorithm for image quality and task activation statistics in high-resolution fMRI.
Theory
The aim in mks-GRAPPA is for each set of kernels to train on and learn a consistent readout polarity and inter-segment difference, such that these differences remain intact after separating collapsed slices13. The remaining differences are removed using slice-specific intra- and inter-segment ghost corrections11,14. For multi-shot interleaved EPI with S segments, the periodicity of any shot-polarity combination is 2×S (Fig. 1c). Therefore, mks-GRAPPA requires 2×S unique sets of kernels, independent of the kernel size.Methods
Experiments were conducted at 7T using a head-only birdcage volume transmit coil and 31-channel receive array. T2*-weighted fMRI data were acquired with VFA-FLEET. To ensure consistent slice profiles across shots, recursively designed SLR excitation pulses were employed14,15. For multi-band (MB) excitation, frequency-shifted pulses were summed9. A CAIPI field-of-view (FOV) shift was incorporated directly into the RF pulses independently across shots as described previously14,16, resulting in an FOV/S CAIPI shift, and obviating gradient blips8.Slice-GRAPPA kernels (single-kernel and multi-kernels) with LeakBlock17 were trained on single-band data that were otherwise identical to the MB data. Image reconstruction followed the pipeline in Fig. 1b14.
To compare the two slice-GRAPPA reconstruction algorithms, normalized root-mean-square-error (NRMSE) and slice leakage17 were computed on retrospectively collapsed single-band data. Temporal signal-to-noise ratio (tSNR) was computed on true MB data acquired under protocols that spanned MB-factors, segmentation factors, in-plane acceleration (R), and slice FOVs. In one subject, a breath-hold challenge was performed18, and activation maps were compared.
Results
Example reconstructions at 0.8-mm isotropic from single-kernel and mks-GRAPPA are displayed in Figure 2. There is reduced noise propagation in mks-GRAPPA, resulting in increased tSNR throughout most brain regions. The whole-brain NRMSE, slice leakage, and tSNR from various protocols in two subjects are shown in Fig. 3. mks-GRAPPA outperformed single-kernel GRAPPA for all acquisitions and metrics: NRMSE decreased 10–20%; leakage decreased 9.5–16%; tSNR increased 3.5–14%.Figure 4 shows activation maps from the breath-hold task. Spatial smoothing at 3-mm full-width-half-maximum was applied to increase the sensitivity to subtle effects—since both reconstructions underwent identical processing, this should not bias the results. Differences between the above-threshold maps are subtle and mean z-scores across the mutually activated regions are not significantly different; however, there is notable artifactual sub-threshold activation in the single-kernel reconstruction that is not present in the multi-kernel maps.
Finally, segmented EPI with SMS is also useful for reduced-TE fMRI, as required for CBV-weighted fMRI19. Figure 5 shows near whole-brain reconstructions using VFA-FLEET with TE=14ms at 1-mm isotropic in a patient who had received an injection of the contrast agent, Ferumoxytol20. Improvements in tSNR are less pronounced (1.8%), however, since the large slice coverage improves the acceleration performance of both algorithms21.
Discussion and Conclusion
We have presented mks-GRAPPA, a novel slice-GRAPPA reconstruction algorithm that accounts for static signal discontinuities within and across segments in multi-shot EPI. By training 2×S slice-GRAPPA kernels, rather than one, we demonstrated significant improvements in image quality metrics under a wide range of protocols. SMS will be key for recovering lost temporal efficiency with segmented EPI at high spatial resolution. In this SNR starved regime, mks-GRAPPA allows us to recover a significant portion of lost tSNR.In some cases, mks-GRAPPA did result in increased static ghosting. However, these ghosts tended to be present in the training data, suggesting that in future work, if we apply multi-coil transmission to increase inter-segment slice profile consistency, we can reduce these ghosts. In combination with high-density receive arrays, we will achieve improved undersampling performance22, enabling the push to higher spatial resolution.
Acknowledgements
The authors would like to thank Dr. Mary Kate Manhard and Dr. Congyu Liao for helpful discussions on reconstruction and ghost corrections for SMS; Dr. Anna Blazejewska for assistance with the breath-hold stimulus presentation; and Dr. Jingyuan Chen, Dr. Shahin Nasr, and Mr. Kyle Droppa for assistance with data acquisition.This work was supported in part by the CIHR (MFE-164755), the NIH NIBIB (grants P41-EB030006, R01-EB019437, R01-EB016695), by the BRAIN Initiative (NIH NIMH grants R01-MH111419 and R01-MH111438, and NIBIB grant U01-EB025162), and by the MGH/HST Athinoula A. Martinos Center for Biomedical Imaging; and was made possible by the resources provided by NIH Shared Instrumentation Grants S10-RR023043, S10-RR019371, and S10-OD023637.
References
1. Davids, M., Guérin, B., Malzacher, M., Schad, L. R. & Wald, L. L. Predicting Magnetostimulation Thresholds in the Peripheral Nervous System using Realistic Body Models. Sci. Rep. 7, 1–14 (2017).
2. Olman, C. A. & Yacoub, E. High-field FMRI for human applications: an overview of spatial resolution and signal specificity. Open Neuroimag J 5, 74–89 (2011).
3. Polimeni, J. R. & Wald, L. L. Magnetic Resonance Imaging technology-bridging the gap between noninvasive human imaging and optical microscopy. Curr Opin Neurobiol 50, 250–260 (2018).
4. Butts, K., Riederer, S. J., Ehman, R. L., Thompson, R. M. & Jack, C. R. Interleaved echo planar imaging on a standard MRI system. Magn Reson Med 31, 67–72 (1994).
5. Kim, S. G., Hu, X., Adriany, G. & Ugurbil, K. Fast interleaved echo-planar imaging with navigator: high resolution anatomic and functional images at 4 Tesla. Magn Reson Med 35, 895–902 (1996).
6. Kang, D., Sung, Y. W. & Kang, C. K. Fast Imaging Technique for fMRI: Consecutive Multishot Echo Planar Imaging Accelerated with GRAPPA Technique. Biomed Res Int 2015, 394213 (2015).
7. Chapman, B. et al. Real-time movie imaging from a single cardiac cycle by NMR. Magn Reson Med 5, 246–254 (1987).
8. Setsompop, K. et al. Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magn Reson Med 67, 1210–1224 (2012).
9. Larkman, D. J. et al. Use of multicoil arrays for separation of signal from multiple slices simultaneously excited. J. Magn. Reson. Imaging 13, 313–317 (2001).
10. Nunes, R. G., Hajnal, J. V & Golay, X. Simultaneous slice excitation and reconstruction for single shot EPI. in Proceedings of the 14th Annual Meeting of the ISMRM vol. 14 0293 (2006).
11. Moeller, S. et al. Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain FMRI. Magn. Reson. Med. 63, 1144–1153 (2010).
12. Feinberg, D. A. et al. Multiplexed echo planar imaging for sub-second whole brain FMRI and fast diffusion imaging. PLoS One 5, e15710 (2010).
13. Setsompop, K. et al. Improving diffusion MRI using simultaneous multi-slice echo planar imaging. Neuroimage 63, 569–580 (2012).
14. Berman, A. J. L. et al. Ultra-high spatial resolution BOLD fMRI in humans using combined segmented-accelerated VFA-FLEET with a recursive RF pulse design. Magn. Reson. Med. 85, 120–139 (2021).
15. Kerr, A. B., Pauly, J. M. & Nishimura, D. G. Slice profile stabilization for segmented k-space imaging. in Proceedings of the 3rd Annual Meeting of SMRM 1189 (Proceedings of the Society of Magnetic Resonance in Medicine, 1993).
16. Polimeni, J. R. et al. Rapid multi-shot segmented EPI using the Simultaneous Multi-Slice acquisition method. in Proceedings of the 20th Annual Meeting of ISMRM 2222 (2012).
17. Cauley, S. F., Polimeni, J. R., Bhat, H., Wald, L. L. & Setsompop, K. Interslice leakage artifact reduction technique for simultaneous multislice acquisitions. Magn Reson Med 72, 93–102 (2014).
18. Bright, M. G. & Murphy, K. Reliable quantification of BOLD fMRI cerebrovascular reactivity despite poor breath-hold performance. Neuroimage 83, 559–568 (2013).
19. Kim, S. G. et al. Cerebral blood volume MRI with intravascular superparamagnetic iron oxide nanoparticles. NMR Biomed. 26, 949–962 (2013).
20. Qiu, D., Zaharchuk, G., Christen, T., Ni, W. W. & Moseley, M. E. Contrast-enhanced functional blood volume imaging (CE-fBVI): Enhanced sensitivity for brain activation in humans using the ultrasmall superparamagnetic iron oxide agent ferumoxytol. Neuroimage 62, 1726–1731 (2012).
21. Breuer, F. A. et al. Controlled aliasing in parallel imaging results in higher acceleration (CAIPIRINHA) for multi-slice imaging. Magn Reson Med 53, 684–691 (2005).
22. Uğurbil, K. et al. Brain imaging with improved acceleration and SNR at 7 tesla obtained with 64-channel receive array. Magn. Reson. Med. 82, 495–509 (2019).
Figures
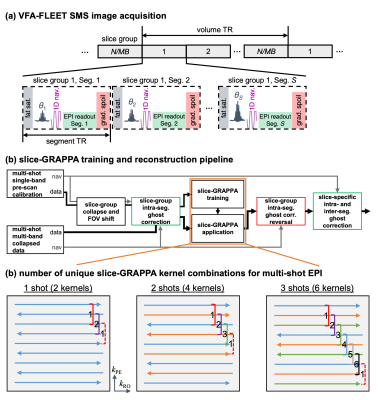
Fig. 1: (a) VFA-FLEET pulse sequence schematic depicting the acquisition of all segments for a given slice group consecutively. (b) The slice-GRAPPA training and image reconstruction pipeline, including tailored slice-specific ghost corrections. (c) Justification of the 2S multi-kernel coverage for multi-shot EPI demonstrated for single-shot, two-shot, and three-shot acquisitions. Shots are represented by different line colours; readout polarities are horizontally offset from each other. A three-line kernel is shown, but this applies to any odd-sized kernel.
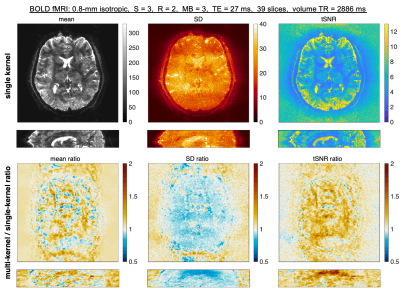
Fig. 2: Animated gif of images from single-kernel slice-GRAPPA or mks-GRAPPA (multi-kernel). The mean, standard deviation (SD), and tSNR across 60 repetitions are displayed from left to right in the top section (alternating between single- and multi-kernel). The ratio of the multi-kernel to single-kernel mean, SD, and tSNR are displayed below. Acquisition parameters are displayed above.
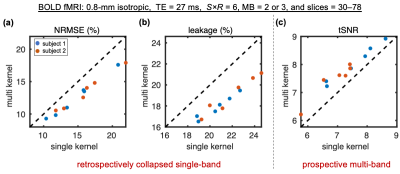
Fig. 3: Comparison of whole-brain normalized RMSE (NRMSE) (a), slice leakage (b), and tSNR (c) between single-kernel (x-axes) and multi-kernel (y-axes) slice-GRAPPA reconstructions. Includes six different protocols acquired at 0.8 mm isotropic in two subjects (blue and orange markers). The combinations of S/R/MB were 2/3/2, 3/2/3, 3/2/2. And for each S/R/MB combination, a smaller slice FOV (30–42 slices) and a larger slice FOV (50–78 slices) were acquired. The dashed lines represent lines of unity.
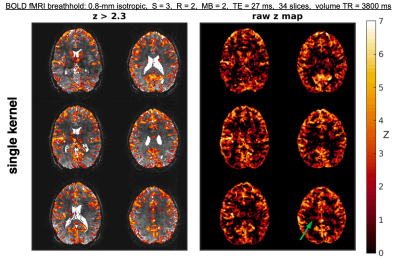
Fig. 4: Animated gif of BOLD activation to a breath-hold challenge alternating between the single- and mks-GRAPPA reconstructions. (left) Uncorrected z-statistic maps (z>2.3) overlayed on the corresponding mean reconstructed images. (right) Unthresholded z-statistic maps. The green arrow in the bottom right shows a region of presumably erroneous, yet coherent, sub-threshold activation in the single-kernel images. This likely arises from unresolved aliasing across slices. The stimulus paradigm consisted of four blocks of 36 s breathing and 15 s breath-hold.
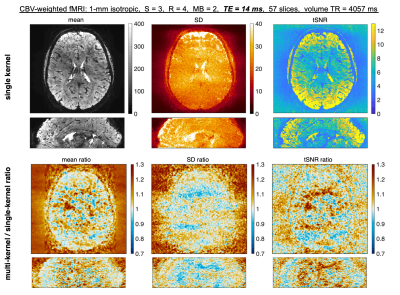
Fig. 5: Animated gif of Ferumoxytol-enhanced Cerebral Blood Volume (CBV)-weighted images from single-kernel slice-GRAPPA or mks-GRAPPA (multi-kernel). The mean, standard deviation (SD), and tSNR across 60 repetitions are displayed from left to right in the top section (alternating between single- and multi-kernel). The ratios of the multi-kernel to single-kernel maps are displayed below. Acquisition parameters are displayed above. Note how the combination of S = 3 and R = 4 (i.e., 12-fold undersampling per shot) was used to achieve a reduced TE of 14 ms, with no partial Fourier.