1102
Accelerated 3D stack-of-spiral bSSFP for functional imaging at 9.4T: Pilot study1Magnetic Resonance Center, Max-Planck Institute for Biological Cybernetics, Tübingen, Germany, 2German Center for Neurodegenerative Diseases (DZNE), Bonn, Germany, 3Department of Biomedical Magnetic Resonance, Eberhard Karls University Tübingen, Tübingen, Germany
Synopsis
We investigate the possibility of using longer multi-shot spiral readouts to accelerate balanced steady-state free precession (bSSFP) at ultra-high field (UHF) for functional imaging. The reconstruction is performed with the full signal model accounting for spatial B0 inhomogeneity and gradient imperfections. We present the activation maps from flickering checkerboard stimulus experiment and discuss challenges of spiral bSSFP at UHF.
Introduction
The BOLD (blood oxygen level dependent) signal of bSSFP sequences is believed to be more localized to the site of neuronal activation because of its higher specificity to smaller vessels1 compared to standard gradient-echo EPI. In addition, the higher BOLD effect and higher SNR at high fields could be used to partially compensate for the loss in sensitivity of the bSSFP technique. These considerations motivate the investigation of bSSFP BOLD at higher fields2. However, the acquisition speed of the conventional Cartesian bSSFP methods is significantly lower than EPI. A Highly segmented echo planar readout with longer TR was proposed for improving the readout efficiency3,4. To further speed up the acquisition, this work looks into the feasibility of a multi-shot spiral bSSFP readout15 for fMRI application at UHF.Methods
Paradigm and fMRI Analysis: An alternating radial checkerboard pattern covering the full visual field is used. The block design consists of equal rest and stimulation duration of 14 s each and 240 volumes were acquired in 5.6 minutes.We used the FSL FEAT package with recommended settings for functional analysis5. A general linear model fitting is used after temporal filtering (30s high pass) with a gamma hemodynamic response function and its temporal derivative as explanatory variables.
Sequence: A highly segmented 3D stack-of-spirals sequence with multiple interleaves is used. The uniform density Archimedean spiral-out trajectory is calculated at the scanner using a time-optimal algorithm6.
Acquisition: Passband bSSFP spiral dataset with TR/TE=10/2 ms, FOV = 192x192x20 mm3, matrix size = 192x192x20 and flip angle= 15 deg is acquired. After in-plane acceleration of R=3, 7x20 spiral acquisitions of about 5.4 ms each are collected which gives a volume TR of 1.4 s. A low resolution triple echo GRE scan is performed to obtain coil sensitivity and spatial B0 field maps. To allow for the correction of gradient imperfections, the gradient impulse response function (GIRF)7 is measured using a 16 channel 19F-NMR field probe system8. All experiments were performed with a human 9.4T scanner (Siemens Healthineers, Erlangen, Germany).
Reconstruction: After retrospectively correcting the trajectory deviations with the measured GIRF, an iterative cg-SENSE reconstruction is performed with NUFFT9 and multi-frequency interpolation (MFI)10 of spatial B0 effects for computational efficiency. The encoding model is preconditioned with the density compensation function (DCF) to reduce the number of iterations required for convergence11. The coil sensitivity maps are calculated using the ESPIRiT method12 of the BART toolbox. The temporal unwarp algorithm called UMPIRE13 followed by denoising by regularized fieldmap estimation14 are used for a robust spatial B0 map computation.
Results
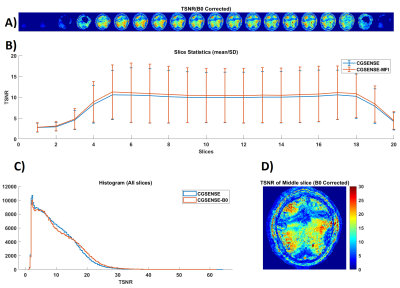
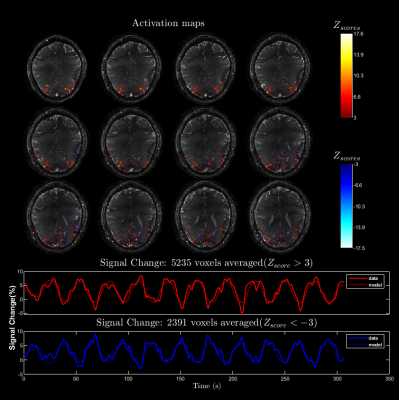
In figure 2, we show the improved image quality of the cg-SENSE reconstruction with B0 correction. Although, the spiral readout was relatively short (~5.4 ms), the spatial B0 inhomogeneity of approximately 200 Hz within the brain results in significant blurring. The tSNR (temporal SNR) analysis in figure 3 shows tSNR values in the range of 15-20 with significantly lower values close to banding artifacts. The spatial B0 correction appreciably increases tSNR across all slices. Even with the sub-optimal experimental condition, the results of our fMRI analysis show considerable positive and negative activations (see Figure 4).Discussion
With a non-optimized ADC duty cycle of 54% and the higher efficiency of spiral readout, we already surpassed the acquisition speed of SE-EPI at lower field4. The major hurdle in sustaining and improving the acquisition speed of bSSFP methods is controlling the spatial B0 inhomogeneity. Some existing solutions to this problem employ advanced shimming techniques or use a phase offset to move the low signal region away from the region of interest when full coverage is not required. Alternatively, phase-cycled bSFFP could be used for full coverage at the cost of spatio-temporal resolution.As the sequence was already operating close to the SAR limit with a flip angle of 15 degrees, no explicit fat suppression was used in the experiment. However, we inverted the slice gradient polarity to move the chemical shift artifact to the top of the acquired volume, so that it shows up as a ring outside of the brain.
Conclusion
The 3D stack-of-spiral acquisition holds potential to enable and accelerate distortion-free accurate functional imaging at UHF with high spatial specificity.Acknowledgements
The financial support of Max-Planck society is gratefully acknowledged.References
1. Scheffler K, Heule R, Báez-Yánez MG, Kardatzki B, Lohmann G. The BOLD sensitivity of rapid steady-state sequences. Magn Reson Med. 2019;81(4):2526-2535. doi:10.1002/mrm.27585
2. Scheffler K, Ehses P. High-resolution mapping of neuronal activation with balanced SSFP at 9.4 tesla. Magn Reson Med. 2016;76(1):163-171. doi:10.1002/mrm.25890
3. Miller KL, Smith SM, Jezzard P, Wiggins GC, Wiggins CJ. Signal and noise characteristics of SSFP FMRI: A comparison with GRE at multiple field strengths. NeuroImage. 2007;37(4):1227-1236. doi:10.1016/j.neuroimage.2007.06.024
4. Ehses P, Scheffler K. Multiline balanced SSFP for rapid functional imaging at ultrahigh field. Magn Reson Med. 2018;79(2):994-1000. doi:10.1002/mrm.26761
5. Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. NeuroImage. 2012;62(2):782-790. doi:10.1016/j.neuroimage.2011.09.015
6. Lustig M, Kim SJ, Pauly JM. A fast method for designing time-optimal gradient waveforms for arbitrary k-space trajectories. IEEE Trans Med Imaging. 2008;27(6):866-873. doi:10.1109/TMI.2008.922699
7. Vannesjo SJ, Haeberlin M, Kasper L, et al. Gradient system characterization by impulse response measurements with a dynamic field camera. Magn Reson Med. 2013;69(2):583-593. doi:10.1002/mrm.24263
8. Barmet C, De Zanche N, Wilm BJ, Pruessmann KP. A transmit/receive system for magnetic field monitoring of in vivo MRI. Magn Reson Med. 2009;62(1):269-276. doi:10.1002/mrm.21996
9. Fessler JA, Sangwoo Lee, Olafsson VT, Shi HR, Noll DC. Toeplitz-based iterative image reconstruction for MRI with correction for magnetic field inhomogeneity. IEEE Trans Signal Process. 2005;53(9):3393-3402. doi:10.1109/TSP.2005.853152
10. Man LC, Pauly JM, Macovski A. Multifrequency interpolation for fast off-resonance correction. Magn Reson Med. 1997;37(5):785-792. doi:10.1002/mrm.1910370523
11. Pruessmann KP, Weiger M, Börnert P, Boesiger P. Advances in sensitivity encoding with arbitrary k -space trajectories: SENSE With Arbitrary k -Space Trajectories. Magn Reson Med. 2001;46(4):638-651. doi:10.1002/mrm.1241
12. Uecker M, Lai P, Murphy MJ, et al. ESPIRiT-an eigenvalue approach to autocalibrating parallel MRI: Where SENSE meets GRAPPA. Magn Reson Med. 2014;71(3):990-1001. doi:10.1002/mrm.24751
13. Robinson S, Schödl H, Trattnig S. A method for unwrapping highly wrapped multi-echo phase images at very high field: UMPIRE. Magn Reson Med. 2014;72(1):80-92. doi:10.1002/mrm.24897
14. Fessler JA, Yeo D, Noll DC. Regularized Fieldmap Estimation in MRI. 2006;(October):706-709. doi:10.1109/isbi.2006.1625014
15. Veldmann, M. et. al. Spiral in/out trajectory with GIRF prediction for passband bSSFP fMRI at 7T, ESMRMB 2020 Meeting.
Figures
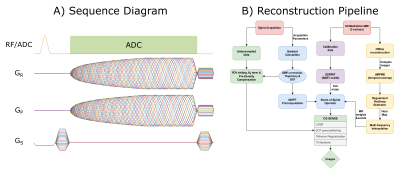
Figure 1: (A) All interleaves of the spiral-out multi-shot bSSFP sequence are shown. The ADC duty cycle of the acquisition used in this study is only about 54%. It could be increased further up to 70% upon further optimization as shown in the figure. (B) Overview of the steps in the reconstruction pipeline. Most of the reconstruction is implemented in MATLAB.
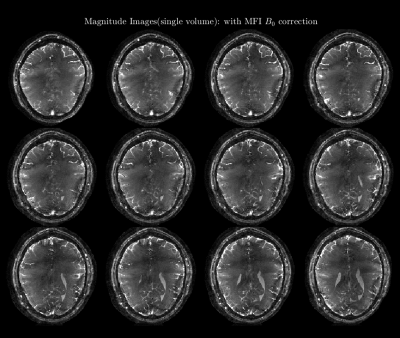
Figure 2: (Animated GIF: click to play) The animation shows the middle slices of a single volume with and without B0 correction. Here, we show both the time and frequency segmentation version of the B0 correction. Both techniques perform equally well. The frequency segmentation method is picked for further analysis due to a slightly smaller computation time.