1092
3D stack-of-stars MR thermometry sequence during hepatic microwave ablation: a phantom study with a commercially available microwave generator
Dominik Horstmann1, Bennet Hensen1, Karen Meyer zu Hartlage1, Daniel Luca Reimert1, Josef Joaquin Löning Caballero1, Frank Wacker1, and Marcel Gutberlet1
1Hannover Medical School, Hannover, Germany
1Hannover Medical School, Hannover, Germany
Synopsis
A 3D stack-of-stars MR-thermometry sequence was developed and evaluated during hepatic microwave ablation in a chicken phantom. A commercially available microwave generator (MWG) working in pulsed mode controlled by a temperature sensor was used. Results show a spatially dependent phase-offset in the MR-image only during active ablation mode of the pulsed MWG impairing thermometry. Consequently, 73% of thermometry data were discarded for the fixed phantom yielding a temperature precision of 0.72°C±0.60°C whereas 94% were discarded for the moving phantom with an accuracy of 1.08°C±1.25°C. For clinical MR-thermometry, an MWG with less EMI is required to improve resolution and temperature precision.
Introduction
The clinical relevance of minimally invasive microwave ablation (MWA) of tumors is constantly rising. Advantages include less heat-sink effect in comparison to radiofrequency ablation, which is beneficial in highly vascular tissues such as the liver. Additionally, microwave antennas offer a very precise delivery of heat and enable large ablation zones. Monitoring MWA via magnetic resonance (MR) thermometry assures a precise knowledge of the size of the ablation zone during treatment via real-time temperature mapping. Low electromagnetic interference (EMI) of the microwave generator with the MR system is a prerequisite for MR thermometry. A commercially available and FDA-approved microwave generator (MWG) allows MR imaging with only small degradation of the SNR when modified with RF chokes and copper wool for grounding1. These modifications do not harm the certificate as medical device of the MWG. Purpose of this work was to implement and evaluate MR thermometry with a 3D stack-of-stars sequence for monitoring hepatic microwave ablation.Methods
3D MR thermometry of MWA was performed in a 1.5T Scanner (MAGNETOM Avanto, Siemens Healthcare, Erlangen, Deutschland) using a stack-of-stars sequence as described by Svedin et al.2 with a golden angle increment between consecutive spokes of θ=38.978°, TE=1.8ms, TR=4ms, spatial resolution of 2.5x2.5x4 mm3 and a field of view of 320x320x32 mm3. To reduce the impairment by breathing motion, DC gating3 was used. The utilized microwave generator system (MedWaves, San Diego, USA) works in pulsed mode with a duty cycle determined by a temperature sensor in the tip of the microwave antenna to reach a pre-set temperature of 100°C. In concordance to Gorny et al.¹, to reduce EMI, the MWG was located outside of the scanner room and the power lines of the microwave applicator as well as of its temperature sensors were introduced through a waveguide into the scanner room. Grounding of all elements and usage of RF chokes allowed further reduction of EMI. To investigate electromagnetic interferences, MR imaging was performed in a water phantom (per 1000g H2O: 1.25g NiSO4 x 6H2O + 5g NaCl) with the microwave applicator inserted in a water filled bottle. Signal-to-noise ratio (SNR) and the image phase of MR thermometry were compared with data acquired before starting the MWG. All data were corrected for phase drift2 and gradient delay4. Furthermore, 3D MR thermometry was performed during MWA in a chicken breast phantom. Breathing motion was simulated by an electric motor moving the phantom. In order to reduce the influence of motion, only data acquired in expiration were used. The accuracy of the measured local temperature was evaluated in comparison to two temperature sensors placed within the chicken phantom.Results
Our setup enables MR imaging with degradation of SNR of 7.3% with the MWG in standby mode and 7.8% with the MWG in ablation mode. Figure 1 shows the DC signal of the water phantom during MWA in a nearby located water filled bottle. The green dots mark spokes sampled before the MWG is turned on (used for the baseline image) while the following spokes are measured with the MWG in ablation mode. The DC signal decreases with the MWG actively ablating and turns back to the baseline level between ablation pulses (red dots). While the phase-offset of the reconstructed image from data in standby mode (figure 2 a) has no spatial dependency with approximately zero mean (µoffset,1=0.000 ± 0.024), for the data reconstructed in active mode a spatially dependent phase offset is shown (figure 2 b) with µoffset,2=0.004 ± 0.195. Both phase images show the phase-offset relative to the baseline scan (green dots in figure 1). Because of the spatially dependent phase offset in active mode, MR thermometry of the chicken phantom was assessed only with spokes sampled in the standby mode between ablation pulses. This leads to discarding 73% of the thermometry data acquired for a fixed chicken phantom and 94% of the data for the moving phantom. Figure 3 a) shows the temperature for one voxel in the fixed chicken phantom and image b) illustrates the corresponding temperature maps for different times of the ablation. A temperature precision of 0.72°C ± 0.60°C at a temporal resolution of 16s and a dice score of 73.4% ± 14.9% were found. Figure 4 shows the corresponding results for the chicken phantom with simulated breathing motion. In this case, a temperature precision of 1.08°C ± 1.25°C at a temporal resolution of 50s and a dice score of about 50% were achieved.Discussion
The results show that the commercially available MWG enables MR imaging with only little SNR degradation. MR Thermometry, more sensitive too EMI, is also feasible but restrictions must be considered: As figure 2 b) indicates, the pulsed MWG produces a spatially dependent phase offset in active mode, which impairs MR thermometry. Therefore, only data in standby mode, which are not impaired by the MWG, may be used for accurate MR thermometry. Consequently, the available data for reconstruction of MR imaging is reduced in moving organs such as the liver. This affects temporal and spatial resolution as well as temperature precision. In conclusion, for clinical use of MR thermometry to monitor hepatic MWA, a MWG with less EMI is mandatory.Acknowledgements
The work of this paper is funded by the Federal Ministry of Education and Research within the Research Campus STIMULATE under the number 13GW0473B.References
- K.R. Gorny, C.P. Favazza, A. Lu, J.P. Felmlee, N.J. Hangiandreou, J.E. Browne, W.S. Stenzel, J.L. Muggli, A.G. Anderson, S.M. Thompson, D.A. Woodrum, Practical implementation of robust MR-thermometry during clinical MR-guided microwave ablations in the liver at 1.5 T, Physica Medica, Volume 67, 2019, Pages 91-99, ISSN 1120-17972.
- Svedin, Bryant T. ; Payne, Allison ; Bolster, Bradley D. ; Parker, Dennis L.: Multiecho pseudo-golden angle stack of stars thermometry with high spatial and temporal resolution using k-space weighted image contrast. In: Magnetic Resonance in Medicine 79 (2018), Nr. 3, S. 1407–1419
- Feng, Li ; Axel, Leon ; Chandarana, Hersh ; Block, Kai T. ; Sodickson, Da- niel K. ; Otazo, Ricardo: XD-GRASP: Golden-angle radial MRI with reconstruction of extra motion-state dimensions using compressed sensing. In: Magnetic Resonance in Medicine 75 (2016), Nr. 2, S. 775–788
- Block, Kai T. ; Uecker, Martin: Simple Method for Adaptive Gradient-Delay Compensation in Radial MRI. In: Proceedings of the 19th Annual Meeting of ISMRM, Montreal, Canada (2011)
Figures
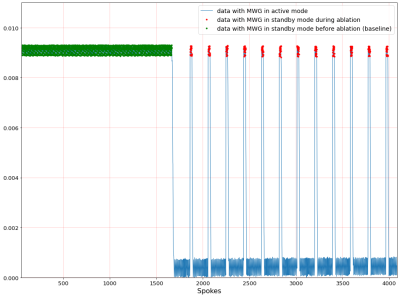
Figure 1: DC signal of water phantom located nearby to ablation object. Green dots indicate the baseline spokes that are sampled while the MWG is turned off. The red dots mark those spokes that are measured between ablation pulses of the MWG. All other spokes refer to active ablation.
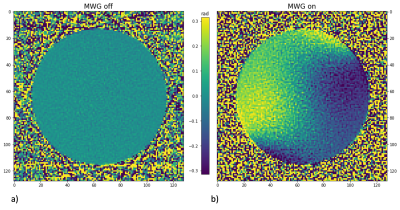
Figure 2: Image a) shows the phase-offset (in rad) of the image reconstructed from spokes that are sampled during ablation with the MWG in standby mode compared to the baseline image (red dots in figure1) whereas image b) results from spokes that are sampled with the MWG in active ablation mode.
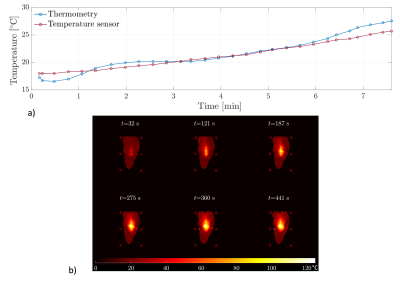
Figure 3: Part a) illustrates the temperature of the fixed chicken phantom while MWA measured via MR thermometry (blue) and via a temperature sensor (red). Part b) shows the corresponding temperature maps for different times during ablation.
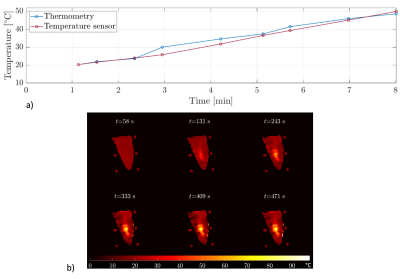
Figure 4: Part a) illustrates the temperature of the moving chicken phantom while MWA measured via MR thermometry (blue) and via a temperature sensor (red). Part b) shows the corresponding temperature maps for different times of the ablation.
DOI: https://doi.org/10.58530/2022/1092