1088
Robust Fiducial-based Registration for Mechatronics-Assisted MRI-guided Focal Laser Ablation of Localized Prostate Cancer1Biomedical Engineering, Western University, London, ON, Canada, 2Robarts Research Institute, London, ON, Canada, 3Medical Biophysics, Western University, London, ON, Canada
Synopsis
MRI-guided focal laser ablation (FLA) is a promising minimally-invasive therapy method for men with localized prostate cancer. We previously developed an MR-compatible mechatronic system capable of transperineal needle delivery within an in-bore 3T MRI environment. This work presents an improved multi-fiducial structure for robust registration of the mechatronic system for MRI-guided FLA needle delivery. Real-time MRI-guided needle delivery was performed in a tissue-mimicking prostate phantom to virtual targets simulating focal ablation zones. With the implementation of the improved multi-fiducial structure, mechatronics-assisted MRI-guided needle delivery enables a 1.44 mm ablation radius, showing potential utility for accurate FLA to small prostate lesions.
Introduction
Multiparametric (mp) MRI of the prostate has been shown to be valuable for the identification of localized prostate cancer, therapy guidance, and monitoring of treatment response.1 With the need for improved treatment options in localized low and intermediate-risk prostate cancers, MRI-guided focal laser ablation (FLA) is a promising minimally-invasive therapy method.2 FLA has demonstrated potential for improved localized cancer control, while preserving delicate anatomical structures and regions of healthy surrounding tissues, reducing adverse side effects and improving the quality of life.3 Highly accurate MR-guided needle delivery is pivotal to the therapeutic success of FLA therapy.We previously developed an MR-compatible mechatronic system capable of remotely actuated transperineal needle positioning within an in-bore 3T MRI environment.4 In our previous work, the mechatronic system was able to align the needle guide to virtual targets at a 10.0 cm depth with an accuracy of 3.84 mm within 95% confidence. However, limitations in fiducial-based localization during registration of the mechatronic system and MRI coordinate system, and possible needle deflections in tissue may limit its clinical performance. In this work, we developed an improved multi-fiducial structure and robust registration method, then performed validation of MR-guided needle delivery to virtual targets simulating localized focal zones in tissue-mimicking phantoms.
Methods
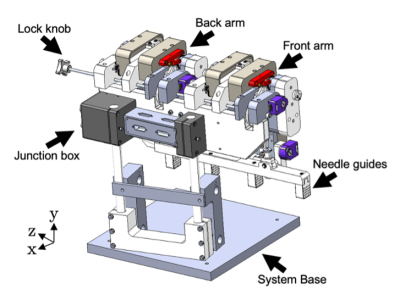
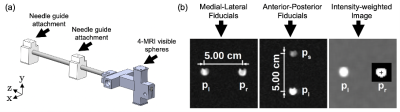
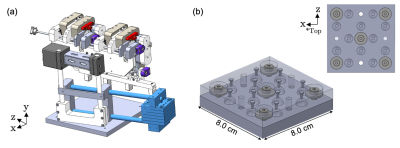
We developed a compact MRI-compatible mechatronic system (Fig. 1) for transperineal FLA of localized prostate cancer with the patient positioned in-bore in dorsal recumbent position.4 The mechatronic system contains three needle trajectory alignment guides suspended between dual-link arms, which are remotely actuated by MR-compatible piezoelectric motors and optical encoders in four degrees-of-freedom: two translational perpendicular to the needle axis and two rotational with a 60x60 mm range-of-motion in each direction. Custom software modules were developed for displaying the acquired MRI images, registration of coordinate systems, operator input and control, and the calculation of inverse and forward kinematics.Registration of the mechatronic system’s needle axis coordinate system and the MR image coordinate system was performed using custom-designed multi-fiducial structures. The previously-designed fiducial structure (Fig. 2) contained four gadolinium-filled MR-visible spheres (Gadovist, 1.0 mmol/mL) in two orthogonal directions. This arrangement was deemed susceptible to fiducial-based localization error, as it required manual alignment of the fiducial with the system axes and used a single fiducial for localizing the endpoints of the anterior-posterior and medial-lateral axes. To address these limitations, an improved multi-fiducial structure (Fig. 3) was designed with 36 gadolinium-filled MR-visible spheres arranged in three layers of 12 spheres. Registration was performed by localizing the intensity-weighted centroid of each fiducial by semi-automatically delineating the spheres with a bounding box, then estimating the transformation parameters using a least-squares fit.
Mechatronic MR-guided needle delivery was assessed with an agar-based tissue-mimicking prostate phantom embedded into a tissue-mimicking background5 to simulate the T1 and T2-weighted relaxation values in human tissues.6 The prostate was positioned 10.0 cm from the surface of the phantom to simulate the anatomical distance from the perineum to prostate and the typical FLA needle insertion depth. T1-weighted gradient-recalled echo (GRE) MR images were acquired on a clinical Discovery MR750 3T MRI scanner (GE Healthcare) with acquisition parameters: FOV = 360x360 mm, in-plane spatial resolution = 1.4×1.4 mm2, slice thickness = 3.0 mm, TR = 270 ms, TE = 4 ms, flip-angle = 25° and bandwidth = 195 Hz pixel-1. Two points on the MR images were selected by the operator as the needle entry and tip position for simulated FLA needle delivery. The system was remotely actuated from the control room, then when positioned, the mechatronic system was locked, and needles were manually inserted to the virtual target, simulating a localized ablation region in a FLA procedure. Post-needle insertion verification MR images were acquired, and the needle track was delineated to compute the Needle Tip Error (as the Euclidian distance) and the Needle Trajectory Error (as the angular separation) between the planned and actual needle trajectory. An upper 95% confidence interval was computed on the Needle Tip Error as the ablation radius.
Results and Discussion
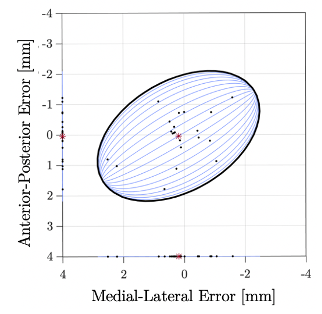
With the improved multi-fiducial registration structure, needle positioning verification to virtual targets in the tissue-mimicking phantom resulted in a mean (SD) Needle Tip Error of 1.13 ± 0.72 mm (N=20) and Needle Trajectory Error of 0.0087 ± 0.0054° (N=20). The spatial distribution of the Needle Tip Error in the anterior-posterior and medial-lateral components are shown in Fig. 3. Within 95% confidence, this error enables an FLA ablation radius of 1.44 mm. These phantom results show that the improved multi-fiducial structure reduces errors in fiducial localization, while accounting for needle deflections in the tissue-mimicking phantom. Importantly, this accuracy sufficiently meets the demand for targeting localized focal zones within a typical 5 mm planned ablation region margin of healthy tissue in clinical practice.7Conclusion
We developed an improved multi-fiducial structure for the robust registration of coordinate systems for MRI-guided FLA therapy. Our results show improved performance of mechatronic MR-guided needle delivery in a tissue-mimicking phantom to simulated FLA ablation zones 2.88 mm in diameter. Current work is focused on increasing the number of MRI-guided needle delivery experiments in prostate phantoms and providing a comprehensive evaluation of needle positioning and delivery error prior to a clinical feasibility study.Acknowledgements
The authors gratefully acknowledge the funding support from the Natural Sciences and Engineering Research Council of Canada (NSERC), the Canadian Institutes for Health Research (CIHR), the Ontario Research Fund–Research Excellence Program (ORF-RE) and the Ontario Institute for Cancer Research (OICR) Imaging Program.References
[1] Ghai, S., & Haider, M. A. (2015) Multiparametric-MRI in diagnosis of prostate cancer. Indian Journal of Urology, Journal of the Urological Society of India, 31(3), 194–201.
[2] Mehralivand, S., George, A. K., Hoang, A. N., Rais-Bahrami, S., Rastinehad, A. R., Lebastchi, A. H., Ahdoot, M., Siddiqui, M. M., Bloom, J., Sidana, A., Merino, M. J., Choyke, P. L., Shih, J. H., Turkbey, B., Wood, B. J., & Pinto, P. A. (2021) MRI-guided focal laser ablation of prostate cancer: a prospective single-arm, single-center trial with 3 years of follow-up. Diagnostic and Interventional Radiology (Ankara, Turkey), 27(3):394–400.
[3] Van Tol-Geerdink, J. J., Leer, J. W., van Oort, I. M., van Lin, E. J., Weijerman, P. C., Vergunst, H., Witjes, J. A., & Stalmeier, P. F. (2013) Quality of life after prostate cancer treatments in patients comparable at baseline. British Journal of Cancer, 108(9), 1784–1789.
[4] Knull, E., Bax, J. S., Park, C. K. S., Tessier, D., & Fenster, A. (2021) Design and validation of an MRI-compatible mechatronic system for needle delivery to localized prostate cancer. Medical Physics, 48(9), 5283–5299.
[5] Rickey, D. W., Picot, P. A., Christopher, D. A., & Fenster, A. (1995) A wall-less vessel phantom for Doppler ultrasound studies. Ultrasound in Medicine and Biology, 21(9), 1163–1176.
[6] Lindner, U., Lawrentschuk, N., Weersink, R. A., Raz, O., Hlasny, E., Sussman, M. S., Davidson, S. R., Gertner, M. R., & Trachtenberg, J. (2010) Construction and evaluation of an anatomically correct multi-image modality compatible phantom for prostate cancer focal ablation. The Journal of Urology, 184(1), 352–357.
[7] Knull, E., Oto, A., Eggener, S., Tessier, D., Guneyli, S., Chatterjee, A., & Fenster, A. (2019) Evaluation of tumor coverage after MR-guided prostate focal laser ablation therapy. Medical Physics, 46(2), 800–810.
Figures