1081
Functional changes in GABA during motor learning with GABA-editing at 3T
Tiffany K Bell1,2,3, Alexander R Craven4,5, Kenneth HugDahl4,6,7, Ralphe Noeske8, and Ashley D Harris1,2,3
1Department of Radiology, University of Calgary, Calgary, AB, Canada, 2Hotchkiss Brain Institute, University of Calgary, Calgary, AB, Canada, 3Alberta Children's Hospital Research Institute, University of Calgary, Calgary, AB, Canada, 4Department of Biological and Medical Psychology, University of Bergen, Bergen, Norway, 5Department of Clinical Engineering, Haukeland University Hospital, Bergen, Norway, 6Division of Psychiatry, Haukeland University Hospital, Bergen, Norway, 7Department of Radiology, Haukeland University Hospital, Bergen, Norway, 8GE Healthcare, Berlin, Germany
1Department of Radiology, University of Calgary, Calgary, AB, Canada, 2Hotchkiss Brain Institute, University of Calgary, Calgary, AB, Canada, 3Alberta Children's Hospital Research Institute, University of Calgary, Calgary, AB, Canada, 4Department of Biological and Medical Psychology, University of Bergen, Bergen, Norway, 5Department of Clinical Engineering, Haukeland University Hospital, Bergen, Norway, 6Division of Psychiatry, Haukeland University Hospital, Bergen, Norway, 7Department of Radiology, Haukeland University Hospital, Bergen, Norway, 8GE Healthcare, Berlin, Germany
Synopsis
Functional magnetic resonance spectroscopy (fMRS) of GABA at 3T is challenging due to the difficulties of measuring GABA levels; GABA is present in the brain at relatively low concentrations and its signal is overlapped by higher concentration metabolites. Here we use a continuous MEGA-PRESS acquisition to show changes in GABA levels measured in the sensorimotor cortex during motor learning and during a control button pressing task. This indicates GABA levels can be tracked during a functional experiment, allowing fMRS of GABA to be accessible at 3T, the more commonly used field strength.
Introduction
GABA is the major inhibitory neurotransmitter in the brain and plays a key role in motor learning. GABA levels measured using proton magnetic resonance spectroscopy (MRS) at rest correlate with motor learning performance.1 However, resting measurements provide little information regarding GABA dynamics.Functional MRS (fMRS) provides a dynamic measure of neurochemistry changes in response to the presentation and processing of stimuli.2 Although fMRS of Glutamate has been used in several studies, there has been little development of this method for GABA due to the challenges of measuring GABA at 3T. GABA is present in the brain at relatively low concentrations and its signal overlaps with higher concentration metabolites. One option to resolve GABA is to use a higher magnetic field strength (i.e., 7T). This increases SNR and spectral resolution, facilitating metabolite separation and quantification.3 Using 7T fMRS, GABA levels were shown to decrease specifically during motor learning (and not during a control task).1 Though the use of 7T is appealing, access is limited. For GABA fMRS to be widely accessible, it is essential to develop this method at 3T.
GABA can be quantified at 3T using Mescher-Garwood PRESS (MEGA-PRESS).4 Briefly, J-coupling within the GABA molecule is exploited to modulate the GABA signal without affecting other metabolites. A difference spectrum is generated in which overlapping resonances are removed to facilitate GABA quantification.
The aim of this study is to validate fMRS of GABA at 3T. GABA was measured continuously using MEGA-PRESS whilst participants performed a serial reaction time task either with or without a learning component. GABA was expected to decrease during motor learning only.1
Methods
Data was collected from nine healthy right-handed participants (18-40y) scanned twice, one-week apart in a cross-over design, performing both a learning and a random (control) condition with no learning component.A serial reaction time task was used for both conditions in which participants were visually instructed to press one of four buttons, as described in ref1. In the learning condition, a 16-item button pressing sequence was continuously repeated, with participants explicitly informed to expect a repeating sequence. In the random condition, cues were pseudo-randomised, and participants were explicitly told not to expect a sequence. The order of the conditions was counterbalanced across subjects.
Data was collected at 3T (GE MR750w) with a 32-channel head coil. A T1-weighted image (BRAVO) was acquired for voxel placement. The MRS voxel (2.5x2.5x2.5 cm3) was placed in the left sensorimotor cortex (Figure 1A). MRS data was continuously acquired throughout the task (35 minutes, 1128 averages) using MEGA-PRESS (14 ms editing pulses; ON/OFF=1.9/7.46 ppm; TR/TE=1800/68 ms). With these acquisition parameters, the GABA signal is contaminated by macromolecules and henceforth will be referred to as GABA+.
Behavioural and MRS data were averaged into six blocks per subject. The median reaction time was calculated for each block. MRS spectra were analysed using Gannet 3.2.5 GABA+ was quantified relative to creatine (GABA+/Cr). Data quality was assessed using visual inspection.
Behavioural and MRS data were analysed using separate two-way repeated measures ANOVAs, with condition (learning or random) and time (block 1-6) as within-participant factors. Contrasts were used to compare each block with block 1.
Results
One participant was excluded from analyses due to data quality, leaving eight participants.Reaction Time: There was a significant effect of condition (F(1,7)=5.81, p=0.04) and block (F(5,17.6)=16.90, p<0.001), and a significant condition x block interaction effect (F(1.7,11.6)=7.872, p=0.009, Greenhouse-Geisser correction) on reaction time. Reaction time significantly decreased during the motor learning task, but not during the control task (Figure 2A).
GABA+/Cr: There was no significant effect of condition (F(1,7)=0.969, p=0.358), a significant effect of block (F(5,23.8)= 2.68, p=0.037) and no significant condition x block interaction effect (F(5,11.6)=17.6, p=0.953) on GABA+/Cr levels (Figure 2B).
Discussion
We found a significant effect of task block on GABA+ levels, but no significant condition x block interaction effect, suggesting GABA+ levels changed both during motor learning and the control task. This did not agree with our original hypothesis that GABA+ levels would change during motor learning only.Reaction time significantly decreased during the motor learning task, but not during the control task, which indicates participants successfully learnt the motor learning task, whereas no learning took place during the control task (evident by the stable reaction time). Therefore, a lack of motor learning does not explain these unexpected results.
In contrast to Kolasinski et al., who found no change in GABA levels during a button pressing task with no learning component,1 Chen et al. demonstrated a significant drop in GABA levels during a ten minute period of hand-clenching (in which no motor learning took place).6 Indeed, even during the control button pressing task, it is likely that participants will need to inhibit their actions when they are not congruent with the task instructions. Therefore, it is perhaps unsurprising that GABA+ levels would change during both tasks.
More generally, we show it is possible to use fMRS at 3T to track GABA+ levels during a behavioural task. Though further refinement and validation of this method is needed, this is the first step in using fMRS at 3T to probe GABA levels in both healthy cognition and clinical disorders.
Acknowledgements
Funding for this study was provided by the Alberta Children’s Hospital Research Institute University of Calgary, and the Child and Adolescent Imaging (CAIR) Program, University of Calgary, Calgary, Canada.References
- Kolasinski, J. et al. The dynamics of cortical GABA in human motor learning. J Physiol 597, 271–282 (2019).
- Jelen, L. A., King, S., Mullins, P. G. & Stone, J. M. Beyond static measures: A review of functional magnetic resonance spectroscopy and its potential to investigate dynamic glutamatergic abnormalities in schizophrenia. J. Psychopharmacol. 32, 497–508 (2018).
- Pradhan, S. et al. Comparison of single voxel brain MRS AT 3T and 7T using 32-channel head coils. Magn. Reson. Imaging 33, 1013–1018 (2015).
- Mescher, M., Merkle, H., Kirsch, J., Garwood, M. & Gruetter, R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 11, 266–272 (1998).
- Edden, R. A. E., Puts, N. A. J., Harris, A. D., Barker, P. B. & Evans, C. J. Gannet: A Batch-Processing Tool for the Quantitative Analysis of Gamma-Aminobutyric Acid–Edited MR Spectroscopy Spectra. J Magn Reson Imaging 40, 1445–1452 (2014).
- Chen, C. et al. Activation induced changes in GABA: Functional MRS at 7 T with MEGA-sLASER. Neuroimage 156, 207–213 (2017).
- Rae, C. D. A guide to the metabolic pathways and function of metabolites observed in human brain 1H magnetic resonance spectra. Neurochem. Res. 39, 1–36 (2014).
Figures
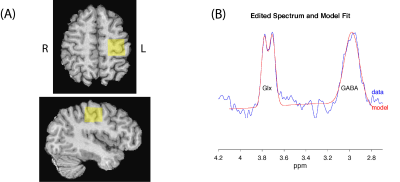
Figure 1(A): Location of the MRS voxel (2.5 x 2.5 x 2.5 cm3) in the left sensorimotor cortex. (B) Data and model fit from a single task block (196 averages).
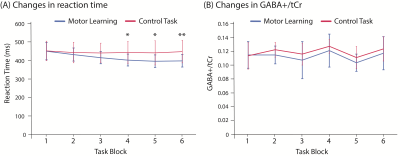
Figure 2: Changes in (A) reaction time and (B) GABA+/Cr levels during the motor learning task (blue) and the control task (red). Error bars represent standard deviation. * denotes blocks in which there is a significant block x condition interaction effect when compared to block 1 (* p<0.05, ** p<0.01).
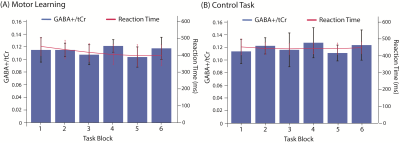
Figure 3: GABA+/Cr levels (shown in blue bars, left axis label) and reaction time (shown as red line and right axis) during (A) the motor learning task and (B) the control task.
DOI: https://doi.org/10.58530/2022/1081