1079
Reproducibility of localized 31P-MRS methods using different surface coils for assessment of hepatic metabolism in vivo at 3T1Institute for Clinical Diabetology, German Diabetes Center, Leibniz Institute for Diabetes Research at Heinrich Heine University, Düsseldorf, Germany, 2German Center for Diabetes Research (DZD e.V.), München-Neuherberg, Germany, 3Department of Endocrinology and Diabetology, Medical Faculty, Heinrich Heine University, Düsseldorf, Germany, 4Department of Radiology and Nuclear Medicine, Maastricht University Medical Center, Maastricht, Netherlands
Synopsis
We determined day-to-day variation and intra-session reproducibility of 31P-MRS-based hepatic phosphorus metabolite quantification. To this end, we compared a standard single loop with a custom-created quadrature surface coil. Absolute concentrations of γ-adenosine triphosphate and inorganic phosphate were assessed in 7 volunteers with both coils. Both coils showed comparable day-to-day and intra-session CVs < 10 %. Metabolite concentrations determined by the two coils were similar. Therefore, both coils yield robust absolute concentrations of hepatic phosphorous metabolites. The quadrature coil allows measurements at a higher distance from the coil which can be important for the application in obese patients.
Introduction
Noninvasive quantification of phosphorus metabolites in the liver is established as a valuable tool to investigate energy metabolism and we previously showed that type 2 diabetes mellitus (T2DM) patients are characterized by lower hepatic adenosine triphosphate (ATP) and inorganic phosphate (Pi) contents, compared to healthy humans1,2. The majority of T2DM patients is overweight or obese, making the determination of hepatic phosphorous metabolites challenging due to a high distance of the region of interest (liver) from the coil and limited sensitivity of standard single loop coils. For this reason, a custom created quadrature coil was designed to perform 31P-magnetic resonance spectroscopy (MRS) in the liver and compared to a multi-purpose standard single loop coil.Thus, the aim of this study was to assess the reproducibility of 31P-MRS measurements using two different coils: the curved quadrature surface coil (RAPID Biomedical, Rimpar, Germany) and a flat circular surface coil (Philips Healthcare, Best, Netherlands).
Methods
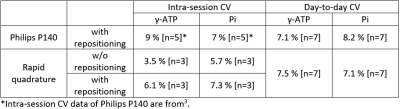
Seven healthy volunteers (6 male/1 female) aged 27 to 56 years (mean ± standard deviation (SD) 38.0 ± 11.6 years; body mass index (BMI) 28.3 ± 3.6 kg/m2; (range: 22.2 to 32.4 kg/m2)) participated in the study.All volunteers underwent measurements with both coils on three different days within a week (4-7 days) in order to assess the day-to-day variation. In order to achieve standardized physiological conditions, each volunteer was measured at the same time of the day after a 4.5 h fast. To assess intra-session variation (reproducibility) of the quadrature coil, a group of 3 volunteers were examined multiple times in a single session. For this, the MRS protocol was performed three times in a row to assess reproducibility without coil repositioning followed by two more measurements where the volunteer has left the scanner briefly to evaluate reproducibility with coil repositioning.
All examinations were performed on a clinical 3-T MR system (3T Philips Achieva dStream, Best, Netherlands). Two different surface coils were used: a flat 14 cm circular, single loop surface coil (transmit-receive coil; Philips Healthcare, Best, Netherlands) and a custom created curved quadrature surface coil (transmit-receive coil; RAPID Biomedical, Rimpar, Germany) with total 31P loop size of 220 mm x 160 mm. The 1H body coil was used for imaging, 1H decoupling and Nuclear Overhauser enhancement (NOE).
Liver spectra were obtained using a 3D localized Image-Selected In vivo Spectroscopy (ISIS) sequence with a 5.42 ms and 3.83 ms hyperbolic secant (HS) adiabatic pulse for excitation (excitation bandwidth 1.15 kHz and 1.63 kHz) for Philips and Rapid, respectively, TR 6 s, number of signal averages (NSA) 128, total acquisition time 13 min, samples (N) 2048, spectral bandwidth (BW) 3 kHz, broadband decoupling (WALTZ-4) and continuous wave (CW) NOE. No breathing trigger was used during both shimming and signal acquisition. Typical line width of the automatic Philips shimming procedure was ~30 Hz.
Spectra of the external reference methylphosphonic acid (MeP) were acquired in separate scans using a pulse-acquire sequence with the identical HS adiabatic pulse of the corresponding coil for excitation, TR 8 s, NSA 16, total acquisition time ~2 min, N 8192, BW 6 kHz, no proton decoupling or NOE was applied.
Absolute concentrations were measured by using the phantom-replacement method3, using in-house mixed phantom containing 50 mM potassium phosphate monobasic (KH2PO4, Sigma Aldrich, Schnelldorf, Germany) and 0.1 mM diethylenetriaminepentaacetic acid (DTPA) (cylindrical phantom, volume 3 l, diameter 16 cm, height 19 cm, T1 10.36 s). Spectra from the phantom were acquired with the same acquisition parameters as in vivo. Corrections were applied for coil loading by measuring the external reference, T1 relaxation times by using the reported values at 3T by Schmidt et al4, the excitation profile of the adiabatic pulse and B1 inhomogeneities of the surface coil in all three spatial directions3.
All liver spectra were processed using jMRUI software (15 Hz Gaußian apodization, zero order phasing) with the AMARES algorithm together with in house established prior knowledge.
Statistical analysis for changes in γ-ATP and Pi of day-to-day-/intra-session variability and test for difference of both coils was performed with a paired t-test.
Results
Table 1 summarizes the data of day-to-day- and intra-session coefficient of variation (CV) of hepatic γ-ATP and Pi of both coils. The absolute concentrations of γ-ATP and Pi was 2.29 ± 0.24 mM and 1.75 ± 0.25 mM for Philips P140 and 2.19 ± 0.23 mM and 1.72 ± 0.20 mM for Rapid quadrature coil, respectively (Table 2). Paired t-test showed no statistical differences in phosphorus metabolites concentrations, determined with the two coils.Discussion and Conclusion
Single voxel (ISIS) 31P-MRS allowed robust detection of γ-ATP and Pi concentrations within a reasonable time frame. Good reproducibility was shown with both coils (CV < 10 %) and determining the metabolites on another day did not introduce additional variation. There was no benefit of the bigger coil loops of the quadrature coil for lean healthy volunteers, however, using the quadrature coil may be beneficial for measurements of people with BMI > 30 kg/m2. The similarity of the metabolite concentrations, measured with the two coils shows the robustness of the phantom-replacement method for absolute quantification.Acknowledgements
No acknowledgement found.References
1. Szendroedi J, Chmelik M, Schmid AI, et al. Abnormal hepatic energy homeostasis in type 2 diabetes. Hepatology 2009;50:1079–1086.
2. Gancheva S, Bierwagen A, Kaul K, et al. Variants in genes controlling oxidative metabolism contribute to lower hepatic ATP independent of liver fat content in type 1 diabetes. Diabetes 2016;65:1849–1857.
3. Laufs A, Livingstone R, Nowotny B, et al. Quantitative liver 31P magnetic resonance spectroscopy at 3T on a clinical scanner. Magnetic Resonance in Medicine 2014;71:1670–1675.
4. Schmid AI, Chmelík M, Szendroedi J, et al. Quantitative ATP synthesis in human liver measured by localized 31P spectroscopy using the magnetization transfer experiment. NMR in Biomedicine 2008;21:437–443.