1075
On the experimental setup of parahydrogen-induced polarization via proton exchange.1Section Biomedical Imaging, Molecular Imaging North Competence Center (MOIN CC), Department of Radiology and Neuroradiology, University Medical Center Schleswig - Holstein, Kiel University, Kiel, Germany
Synopsis
The PHIP-X methodology is a novel approach to hyperpolarize nuclei from specific molecules that could function as contrast agents in metabolic MRI. PHIP-X combines the strong polarization of hydrogenative PHIP with the broad applicability of proton exchange and opens up the variety of potential hyperpolarizable biomolecules. The presented experimental setup facilitates the application of pH2 pressures up to 30 bar and well-defined external magnetic fields up to 80 mT. Using this setup allowed to hyperpolarize glucose (830 mM) and achieved increasing polarization with increasing the pressure from 10 bar to 20 bar (molar polarization was more than doubled).
Introduction
MRI with hyperpolarized contrast agents enabled a wide variety of new applications for medical diagnosis.1 One approach to create strongly hyperpolarized nuclei was parahydrogen induced polarization (PHIP)2, where parahydrogen (pH2), and thus its spin-order, was added to a target molecule by catalytic hydrogenation. However, the pool of hyperpolarizable target molecules by hydrogenative PHIP was limited as an unsaturated C-C bond was needed.Recent advances on the polarization transfer enabled the polarization of many different molecules using pH2 and reversible exchange.3,4 In a novel approach, the strong polarization of PHIP was combined with the broad applicability of proton exchange (PHIP-X)5. Here, a transfer agent was polarized by addition of pH2, and the polarization was transferred to a target molecule. Thus, 1H and 13C polarization were observed. Simulations, however, suggested an increased polarization using a stronger field and higher pressures.5
Here, we present a setup for PHIP-X that allowed much higher pressures and fields than before. By design, the pressurized pH2 acted as the driving force for the sample transfer into the NMR spectrometer. By reaching up to 30 bar and 80 mT, a hyperpolarization of glucose was observed.
Methods
Sample preparation8 µl of Propargyl alcohol (99%, Sigma Aldrich, CAS: 107-19-7), 100 µl of 833 mM D-Glucose-13C6 (Sigma Aldrich, CAS: 110187-42-3), dissolved in Dimethylsulfoxid-d6 (DMSO-d6, Sigma Aldrich, CAS: 2206-27-1), and 1 ml of 7 mM catalyst ([Rh(dppb)(COD)]BF4, 98%, Sigma Aldrich, CAS: 79255-71-3), dissolved in acetone-d6 (Sigma Aldrich, CAS: 666-52-4) were mixed.
The polarizer
A resistive magnet was constructed (1 mm copper wire, 5200 windings) around a 26 cm copper tube, which functioned as a reaction chamber and could withstand pressures of more than 40 bar. A power supply generated fields up to 80 mT at 30 V and 3 A (F71 Teslameter, Lake Shore Cryotronics). A manifold (MV1) beneath the reaction chamber was connected to a pH2 supply, an exhaust, and an NMR tube (Fig. 1). A screw top allowed to inject the sample, and a pressure regulator controlled the pH2 pressure. 51 % pH2 was produced at liquid nitrogen temperatures. 6
The experiment
After setting the magnetic field and injecting the sample, the reaction chamber was closed. For commencing PHIP-X, pH2 was injected at the desired pressure by manually operating MV1. After the reaction time of 2 to 3 seconds, the sample was transferred to a 5 mm NMR tube inside the NMR system. NMR was acquired using a 90° pulse in a 1 T benchtop NMR about 2 s after the beginning of the sample transfer to the NMR.
Results
By increasing the pH2 pressure in the reaction chamber, the hydrogenation yield from the precursor (1) to the transfer agent (2) was increased drastically (Fig. 2). At 1 bar, for ~ 80 mM initial concentration of the precursor, only single hydrogenation to 2a was observed after 20 s at 1 bar (~9 %, Fig. 2, bottom left). At 30 bar, however, completed double hydrogenation was observed. When the experiment was repeated for ~ 350 mM concentration of the precursor, at 1 bar about 2 % of 2a were observed after 25 s. With an increased pressure to 30 bar, 25 % of 2a, and 65 % of 2b were observed (Fig. 2, bottom right).Consecutively, we attempted to transfer polarization to glucose. Propargyl alcohol and a catalyst were mixed with 830 mM D-Glucose-13C6, dissolved and subjected to 51 % pH2 for 2 to 3 s at 80 mT. After transfer to the NMR, 13C spectra were acquired, showing enhanced resonances (Fig. 3). A pressure of 10 bar generated a polarization of 0.0010 % and a molar polarization of 0.85 mM·%. With 20 bar, the polarization was 0.0024 % and the molar polarization was 1.99 mM·%. This showed that doubling the pH2 pressure led to a more than doubled polarization enhancement. Note, that the concentration of the target molecule glucose was high with 830 mM. If we decreased the concentration of glucose down to 43 mM, the polarization increased to 0.053 % at 30 bar.5
Discussion
The presented setup could execute PHIP-X experiments at pressures up to 30 bar and external magnetic fields of 80 mT in combination with a sample transfer within ~2 s to the measuring site. It was shown that an increase of the pressure from 1 bar to 30 bar increased the hydrogenation yield drastically. This can be used to achieve high concentrations of transfer agents.Using the presented setup allowed to hyperpolarize glucose (830 mM) and achieved increasing polarization by increasing the pressure from. Note that no dedicated method for spin order transfer was applied and that the enhancements were achieved by spontaneous evolution only.
One challenge for future applications in metabolic MRI is the catalyst and the precursor molecule remaining in the sample solution after the reaction. We plan to develop methods to remove the catalyst and the transfer agent after the reaction.
Conclusion
The presented experimental setup is capable of pressures up to 30 bar and can be used to generate a high molar polarization of respective target molecules. As a successful proof-of-concept, this setup can be the basis for future developments towards the production of hyperpolarized media.Acknowledgements
The work is supported by the Emmy Noether Program "metabolic and molecular MR" (HO 4604/2-2),. Kiel University and the Medical Faculty are acknowledged for supporting the Molecular Imaging North Competence Center (MOIN CC) as a core facility for imaging in vivo. MOIN CC was founded by a grant from the European Regional Development Fund (ERDF) and the Zukunftsprogramm Wirtschaft of Schleswig-Holstein (Project no. 122-09-053).References
[1] Skinner, J.G., Menichetti, L., Flori, A. et al. Metabolic and Molecular Imaging with Hyperpolarised Tracers. Mol Imaging Biol 20, 902–918 (2018). https://doi.org/10.1007/s11307-018-1265-0.
[2] C. Russell Bowers and D. P. Weitekamp: Parahydrogen and synthesis allow dramatically enhanced nuclear alignment. J. Am. Chem. Soc. 1987, 109, 18, 5541–5542.
[3] Wissam Iali, Peter J. Rayner and Simon B. Duckett: Using parahydrogen to hyperpolarize amines, amides, carboxylic acids, alcohols, phosphates, and carbonates. Sci. Adv. 2018; 4: eaao6250.
[4] Soumya S. Roy, Kate M. Appleby, Elizabeth J. Fear, and Simon B. Duckett: SABRE-Relay: A Versatile Route to Hyperpolarization. The Journal of Physical Chemistry Letters 2018 9 (5), 1112-1117 DOI: 10.1021/acs.jpclett.7b03026.
[5] Kolja Them, Frowin Ellermann, Andrey N. Pravdivtsev, Oleg G. Salnikov, Ivan V. Skovpin, Igor V. Koptyug, Rainer Herges, and Jan-Bernd Hövener: Parahydrogen-Induced Polarization Relayed via Proton Exchange. J. Am. Chem. Soc. 2021, 143, 34, 13694–13700.
[6] Frowin Ellermann, Andrey Pravdivtsev, and Jan-Bernd Hövener: Open-source, partially 3D-printed, high-pressure (50-bar) liquid-nitrogen-cooled parahydrogen generator. Magn. Reson., 2, 49–62, 2021.
Figures
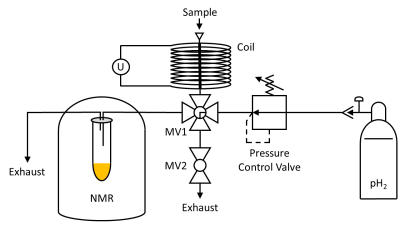
Figure 1: Schematic view of the experimental setup developed for PHIP-X. A valve (MV1) was connecting the pH2 supply (right side) with the reaction chamber (top), exhaust, and NMR tube. A pressure regulator between the gas bottle and MV1 allowed setting the pressure. The reaction chamber was surrounded by a resistive magnet (NGL 202 Power Supply, Rohde & Schwarz) and featured a closure cap. MV1 established a connection of the reaction chamber with the NMR tube, which sits inside the NMR (Spinsolve 43 Carbon, Magritek) (left side). The setup contained two exhaust tubes to release pressure.
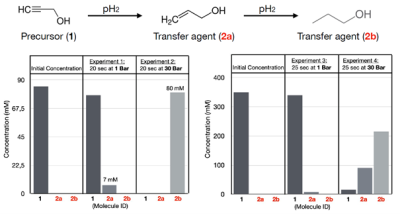
Figure 2: Schematic view of the hydrogenation of the precursor propargyl alcohol (1) to the transfer agents allyl alcohol (2a) and propanol (2b). A hydrogenation pressure of 30 bar led to a hydrogenation yield of 100 % (80 mM precursor concentration, bottom left) and 25+65 %, respectively (350 mM precursor concentration, bottom right), while 1 bar pH2 pressure it was observed a hydrogenation yield to 2a of 9 % and 2 %, respectively. The data was acquired from thermal 1H spectra with about 10 averages.
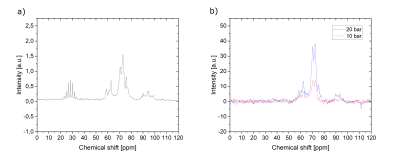
Figure 3: 13C NMR spectra of thermally (a) and hyperpolarized (b) glucose after PHIP-X with 10 and 20 bar (830 mM glucose, 7 mM catalyst, 100µl DMSO-d6, and 1000µl acetone-d6). Both y-scales were multiplied by 100000 and are comparable. The thermal spectrum (a) was acquired with 4000 averages. The resonances between 20 and 40 ppm were caused by the solvent acetone-d6. The hyperpolarized spectra were acquired with 1 average and have a higher SNR than the thermal spectrum. The glucose resonances are located between 60 and 100 ppm in both spectra.