1072
In-vivo Imaging of hyperpolarized and purified Fumarate at 11.7 T1Internal Medicine II, Ulm University Medical Center, Ulm, Germany, 2NVision Imaging Technologies GmbH, Ulm, Germany, 3Department of Nuclear Medicine, Ulm University Medical Center, Ulm, Germany, 4Institute for Pharmacology and Toxicology, Ulm University Medical Center, Ulm, Germany
Synopsis
Hyperpolarized [1-13C] fumarate has been shown to be a reliable imaging biomarker for monitoring cellular necrosis with conversion of [1-13C]fumarate to [1-13C]malate via Fumarate hydratase. However, the inherently low 13C-sensitivity limits biomedical applications with a need for accessible and low-cost hyperpolarization methods.
In this abstract we show that parahydrogen-induced polarization methods can overcome these limitations by providing highly polarized and purified [1-13C]fumarate as a non-toxic perfusion agent with the capabilities of acting as a biomarker of cell necrosis in metabolic 13C MRI.
Introduction
Fumarate, which is converted to malate in one step of the Krebs cycle, may act as a sensitive biomarker for cell necrosis, which has already been demonstrated in pre-clinical studies to image tumor response to therapy1 and acute kidney injury2 or myocardial infarction3. The inherently low NMR sensitivity of 13C requires prior hyperpolarization to achieve sufficient signal intensities for imaging within the lifetime of the hyperpoalrized state. In contrast to DNP methods, PHIP (parahydrogen-induced polarization) methods are easier to implement, an order of magnitude less expensive and polarization can be achieved within seconds.Here, we demonstrate that parahydrogen-polarized fumarate solutions can be efficiently purified of contaminants by acid precipitation4 and subsequent redissolution to facilitate in-vivo application in mice.
Methods
Prior to imaging, the compound was tested for cell toxicity in six human and rodent cell lines at high concentrations (max. 3.2 mM fumarate). In none of the performed experiments any cytotoxic effects were found.Hyperpolarized fumarate was generated by hydrogenation of 13C labelled acetylenedicarboxylic acid with para enriched hydrogen. The polarization of the proton singlet state was converted into 13C magnetization using a linear magnetic field sweep. After cleaning, 100 $$$\mu$$$l of the hyperpolarized 13C fumarate solution was injected intravenously.
Imaging was performed five seconds and ten seconds after injection in one animal each, to allow for circulation of the compound. All data were acquired on a 11.7 T pre-clinical MRI system (BioSpec 117/16 USR, Bruker, Ettlingen, Germany) using an in-house built 13C birdcage coil (figure 1) in combination with a 72 mm inner diameter volume resonator (089/072 Quad to AD, Bruker, Ettlingen, Germany). The 13C-coil has 41 mm in diameter is made of eight legs with each 60 mm in length. The coil base is PLA printed and the coil is tuned and matched inside the 1H resonator. 13C reference power calibration was achieved using a dedicated 13C-enriched urea phantom.
In-vivo perfusion (projection) images were acquired using a true-FISP sequence with an isotropic in-plane resolution of 0.5 x 0.5 mm2 and FOV$$$\,=70\,\text{x}\,35$$$ mm, $$$T_\text{E} = 1.5$$$ ms, $$$T_\text{R} = 3$$$ ms, $$$\alpha = 25^\circ$$$, resulting in a frame time of $$$\approx 140$$$ ms. Images were acquired until depletion of the hyperpolarized phase. 1H overlays were acquired subsequently without moving the animal using a FLASH sequence with an in-plane resolution of 0.25 x 0.25 mm2 and a slice thickness of 1 mm.
Results
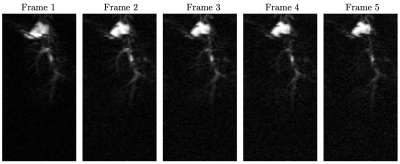
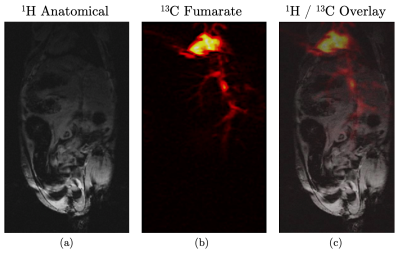
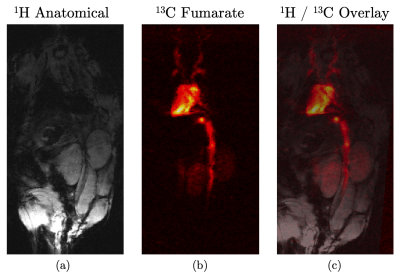
Figure 2 shows five time frames, acquired contiguously within 700 $$$\mu$$$s, starting five seconds after complete injection of hyperpolarized fumarate. Five frames were averaged to yield the 13C fumarate perfusion image as shown in figure 3b). The corresponding anatomical image is shown in 3a). An overlay of the perfusion and anatomical image is shown in figure 3c).The same is shown in figure 4 for the second animal, were the image acquisition started 10 s after injection of the hyperpolarized agent.
Non of the investigated animals showed any side-effect after administration of the purified compound.
The polarization of the hyperpolarized tracer was measured using a 13C benchtop NMR spectrometer after purification, yielding polarization values of about 10 %.
Discussion and Conclusion
The hyperpolarized 13C signal is clearly visible in the inferior vena cava, the heart, the left and right renal veins as well as in the left and right common carotid arteries with a perfusion of the kidneys not later than 10 s after injection.The acquired perfusion images are in good accordance with the anatomical references, showing sufficient SNR even after acquisition of several image frames at an in-plane resolution of 0.5 mm, making hyperpolarized fumarate based on PHIP a cost-efficient and feasible perfusion agent.
The fumarate's $$$T_1$$$ time of at leat 30 s at 11.7 T in combination with the achieved polarization values appear even sufficient for the short-term observation of metabolic processes within the Krebs cycle in necrotic and well perfused environments.
Acknowledgements
The authors thank the Ulm University Centre for Translational Imaging MoMAN for its support.References
[1] Cavallari Eleonora, et al. In-vitro NMR studies of prostate tumor cell metabolism by means of hyperpolarized [1-13C] pyruvate obtained using the PHIP-sah method. Frontiers in oncology 10 (2020): 497.
[2] Clatworthy, Menna R., et al. Magnetic resonance imaging with hyperpolarized [1, 4-13C2] fumarate allows detection of early renal acute tubular necrosis. Proceedings of the National Academy of Sciences 109.33 (2012): 13374-13379.
[3] Miller, Jack J., et al. Hyperpolarized [1, 4-13C2] fumarate enables magnetic resonance-based imaging of myocardial necrosis. JACC: Cardiovascular Imaging 11.11 (2018): 1594-1606.
[4] Knecht, Stephan, et al. Rapid hyperpolarization and purification of the metabolite fumarate in aqueous solution. Proceedings of the National Academy of Sciences 118.13 (2021).
Figures